当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,2-diol dehydration by the radical SAM enzyme AprD4 – a matter of proton circulation and substrate flexibility
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-19 , DOI: 10.1021/jacs.7b10501 Wan-Qiu Liu 1 , Patricia Amara , Jean-Marie Mouesca , Xinjian Ji 1 , Oriane Renoux , Lydie Martin , Chen Zhang 1 , Qi Zhang 1 , Yvain Nicolet
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-19 , DOI: 10.1021/jacs.7b10501 Wan-Qiu Liu 1 , Patricia Amara , Jean-Marie Mouesca , Xinjian Ji 1 , Oriane Renoux , Lydie Martin , Chen Zhang 1 , Qi Zhang 1 , Yvain Nicolet
Affiliation
|
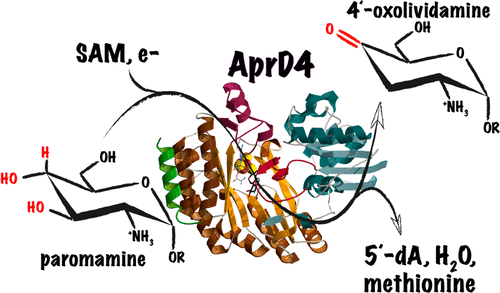
Regiospecific dehydration of vicinal diols by enzymes is a difficult reaction that usually requires activation by dedicated organic cofactors. The enzymatic use of radical-based chemistry is an effective but challenging alternative as radical intermediates are difficult to control. Here we report the X-ray structure of the radical S-adenosyl-l-methionine (SAM) dehydratase AprD4 involved in the biosynthesis of the aminoglycoside (AG) antibiotic apramycin. Using in vitro characterizations and theoretical calculations based on our crystal structure, we have been able to propose a detailed mechanism of AprD4 catalysis, which involves a complex partially substrate-induced proton relay network in the enzyme active site and highlights the key role of the protein matrix in driving high-energy intermediates.
中文翻译:

由自由基 SAM 酶 AprD4 进行的 1,2-二醇脱水——质子循环和底物灵活性的问题
酶对邻二醇的区域特异性脱水是一个困难的反应,通常需要由专用有机辅助因子激活。由于自由基中间体难以控制,基于自由基的化学的酶促使用是一种有效但具有挑战性的替代方法。在这里,我们报告了参与氨基糖苷 (AG) 抗生素安普霉素生物合成的自由基 S-腺苷-l-甲硫氨酸 (SAM) 脱水酶 AprD4 的 X 射线结构。使用基于我们晶体结构的体外表征和理论计算,我们已经能够提出 AprD4 催化的详细机制,它涉及酶活性位点中复杂的部分底物诱导的质子中继网络,并突出了蛋白质的关键作用驱动高能中间体的基质。
更新日期:2018-01-19
中文翻译:

由自由基 SAM 酶 AprD4 进行的 1,2-二醇脱水——质子循环和底物灵活性的问题
酶对邻二醇的区域特异性脱水是一个困难的反应,通常需要由专用有机辅助因子激活。由于自由基中间体难以控制,基于自由基的化学的酶促使用是一种有效但具有挑战性的替代方法。在这里,我们报告了参与氨基糖苷 (AG) 抗生素安普霉素生物合成的自由基 S-腺苷-l-甲硫氨酸 (SAM) 脱水酶 AprD4 的 X 射线结构。使用基于我们晶体结构的体外表征和理论计算,我们已经能够提出 AprD4 催化的详细机制,它涉及酶活性位点中复杂的部分底物诱导的质子中继网络,并突出了蛋白质的关键作用驱动高能中间体的基质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号