Food and Chemical Toxicology ( IF 3.9 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.fct.2018.01.001 Mei Lu 1 , Yuan Jin 1 , Barbara Ballmer-Weber 2 , Richard E Goodman 1
|
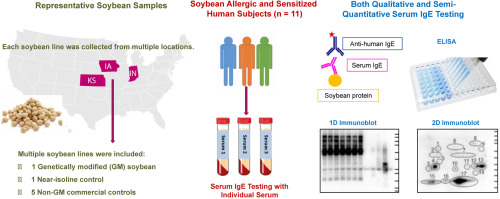
Prior to commercialization, genetically modified (GM) crops are evaluated to determine the allergenicity of the newly expressed protein. Some regulators require an evaluation of endogenous allergens in commonly allergenic crops including soybean to determine if genetic transformation increased endogenous allergen concentrations, even asking for IgE testing using sera from individual sensitized subjects. Little is known about the variability of the expression of endogenous allergens among non-GM varieties or under different environmental conditions. We tested IgE binding to endogenous allergenic proteins in an experimental non-commercial GM line, a non-GM near-isoline control, and five non-GM commercial soybean lines replicated at three geographically separated locations. One-dimensional (1D) and two-dimensional (2D) immunoblotting and ELISA were performed using serum or plasma from eleven soybean allergic patients. The results of immunoblots and ELISA showed no significant differences in IgE binding between the GM line and its non-GM near-isoline control. However, some distinct differences in IgE binding patterns were observed among the non-GM commercial soybean lines and between different locations, highlighting the inherent variability in endogenous allergenic proteins. Understanding the potential variability in the levels of endogenous allergens is necessary to establish a standard of acceptance for GM soybeans compared to non-GM soybean events and lines.
中文翻译:

人类 IgE 与转基因 (GM) 大豆和在多个地点种植的六种非转基因大豆的蛋白质结合的比较研究
在商业化之前,将对转基因 (GM) 作物进行评估,以确定新表达的蛋白质的过敏性。一些监管机构要求对大豆等常见致敏作物中的内源过敏原进行评估,以确定基因转化是否增加了内源过敏原浓度,甚至要求使用个体致敏受试者的血清进行 IgE 检测。对于非转基因品种之间或不同环境条件下内源过敏原表达的变异性知之甚少。我们在一个实验性非商业转基因品系、一个非转基因近等值对照品系以及在三个地理上分离的地点复制的五个非转基因商业大豆品系中测试了 IgE 与内源过敏蛋白的结合。使用来自 11 名大豆过敏患者的血清或血浆进行一维 (1D) 和二维 (2D) 免疫印迹和 ELISA。免疫印迹和 ELISA 结果显示,GM 系与其非 GM 近系对照之间的 IgE 结合没有显着差异。然而,在非转基因商业大豆品系之间以及不同地点之间观察到 IgE 结合模式的一些明显差异,突出了内源性过敏蛋白的固有变异性。了解内源过敏原水平的潜在变异性对于建立转基因大豆与非转基因大豆事件和品系相比的接受标准是必要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号