当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct access to spirobiisoxazoline via the double 1,3-dipolar cycloaddition of nitrile oxide with allenoate†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7ob03040a Xinye Shang 1, 2, 3, 4, 5 , Kun Liu 1, 2, 3, 4, 5 , Zhongyin Zhang 1, 2, 3, 4, 5 , Xianhong Xu 1, 2, 3, 4, 5 , Pengfei Li 5, 6, 7, 8 , Wenjun Li 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7ob03040a Xinye Shang 1, 2, 3, 4, 5 , Kun Liu 1, 2, 3, 4, 5 , Zhongyin Zhang 1, 2, 3, 4, 5 , Xianhong Xu 1, 2, 3, 4, 5 , Pengfei Li 5, 6, 7, 8 , Wenjun Li 1, 2, 3, 4, 5
Affiliation
|
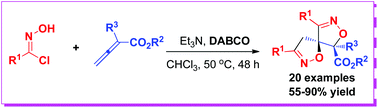
The double [3 + 2]-cycloadditions between nitrile oxides and allenoates have been achieved. In the presence of DABCO combined with Et3N, 2-substituted buta-2,3-dienoates reacted with oxime chlorides to afford spirobiisoxazolines in 55–90% yields via the double 1,3-dipolar cycloaddition. Notably, the construction of double isoxazoline moieties and two chiral centers including a spiro carbon center was achieved.
中文翻译:

通过双氧化氮与脲基酸酯的双1,3-偶极环加成 直接获得螺索比恶唑啉†
已实现了腈氧化物和烯丙酸酯之间的双[3 + 2]-环加成反应。在DABCO与Et 3 N结合存在的情况下,通过双1,3-偶极环加成反应,2-取代的buta-2,3-dienoates与肟氯化物反应,以55-90%的产率提供spirobiisoxazoazolines 。值得注意的是,实现了双异恶唑啉部分和两个包括螺碳中心的手性中心的构建。
更新日期:2018-01-04
中文翻译:

通过双氧化氮与脲基酸酯的双1,3-偶极环加成 直接获得螺索比恶唑啉†
已实现了腈氧化物和烯丙酸酯之间的双[3 + 2]-环加成反应。在DABCO与Et 3 N结合存在的情况下,通过双1,3-偶极环加成反应,2-取代的buta-2,3-dienoates与肟氯化物反应,以55-90%的产率提供spirobiisoxazoazolines 。值得注意的是,实现了双异恶唑啉部分和两个包括螺碳中心的手性中心的构建。











































 京公网安备 11010802027423号
京公网安备 11010802027423号