当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective hydrogenation of CO2 to methanol catalyzed by Cu supported on rod-like La2O2CO3†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7cy01998j Kun Chen 1, 2, 3, 4, 5 , Xinping Duan 1, 2, 3, 4, 5 , Huihuang Fang 1, 2, 3, 4, 5 , Xuelian Liang 1, 2, 3, 4, 5 , Youzhu Yuan 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7cy01998j Kun Chen 1, 2, 3, 4, 5 , Xinping Duan 1, 2, 3, 4, 5 , Huihuang Fang 1, 2, 3, 4, 5 , Xuelian Liang 1, 2, 3, 4, 5 , Youzhu Yuan 1, 2, 3, 4, 5
Affiliation
|
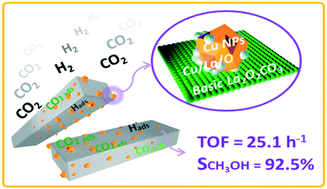
Cu-based catalysts have long been applied to convert CO2 and H2 into methanol, and their performances are well known to be markedly influenced by the support and promoter. Herein, several Cu-based catalysts supported on lanthanum oxides were fabricated by varying the preparation methods and characterized by XRD, TEM, ICP-AES, N2 physisorption, N2O chemisorption, CO2 chemisorption, XPS, and CO2-TPD. The results showed that the as-prepared Cu supported on rod-like La2O2CO3 (La2O2CO3-R) exhibited the highest TOFCu, methanol selectivity and yields of methanol for the hydrogenation of CO2 to methanol. Distinct from conventional promoter addition, the local formation of a new type of Cuδ+ species at Cu/La2O2CO3-R interfaces and the original adsorption performance of basic oxides are the two key factors relating to the catalytic performance. Cu supported on La2O2CO3-R with a higher content of Cuδ+ species and a stronger adsorption for CO2 leads to superior catalytic activities. DRIFTS studies revealed that the generation of synergetic basic sites at the interfacial areas can increase the intrinsic activity of Cu-based methanol catalysts by moderately and selectively stabilizing methanol synthesis intermediates. This work provides new insights into CO2 activation over basic oxide-supported Cu catalysts, and the identification of metal–support interactions between Cu and lanthanum oxides is beneficial for the rational design of stable Cu-based catalysts.
中文翻译:

棒状La 2 O 2 CO 3 †上负载的Cu催化 CO 2选择性加氢成甲醇。
铜基催化剂长期以来一直用于将CO 2和H 2转化为甲醇,众所周知,其性能会受到载体和助催化剂的显着影响。本文中,通过改变制备方法来制备负载在氧化镧上的几种Cu基催化剂,并通过XRD,TEM,ICP-AES,N 2物理吸附,N 2 O化学吸附,CO 2化学吸附,XPS和CO 2 -TPD进行表征。结果表明,负载在棒状La 2 O 2 CO 3(La 2 O 2 CO 3 -R)上的Cu的TOF Cu最高。,甲醇选择性和将CO 2加氢成甲醇的甲醇收率。与常规助催化剂的添加不同,在Cu / La 2 O 2 CO 3 -R界面上局部形成新型Cuδ +物种和碱性氧化物的原始吸附性能是与催化性能有关的两个关键因素。负载在La 2 O 2 CO 3 -R上的Cu具有较高的Cuδ +种类含量和对CO 2的强吸附性导致优异的催化活性。DRIFTS研究表明,在界面区域产生协同碱性位点可以通过适度和选择性地稳定甲醇合成中间体来提高Cu基甲醇催化剂的内在活性。这项工作为碱性氧化物负载的铜催化剂上的CO 2活化提供了新的见解,而铜与镧氧化物之间金属-载体相互作用的鉴定对于稳定铜基催化剂的合理设计是有益的。
更新日期:2018-01-04
中文翻译:

棒状La 2 O 2 CO 3 †上负载的Cu催化 CO 2选择性加氢成甲醇。
铜基催化剂长期以来一直用于将CO 2和H 2转化为甲醇,众所周知,其性能会受到载体和助催化剂的显着影响。本文中,通过改变制备方法来制备负载在氧化镧上的几种Cu基催化剂,并通过XRD,TEM,ICP-AES,N 2物理吸附,N 2 O化学吸附,CO 2化学吸附,XPS和CO 2 -TPD进行表征。结果表明,负载在棒状La 2 O 2 CO 3(La 2 O 2 CO 3 -R)上的Cu的TOF Cu最高。,甲醇选择性和将CO 2加氢成甲醇的甲醇收率。与常规助催化剂的添加不同,在Cu / La 2 O 2 CO 3 -R界面上局部形成新型Cuδ +物种和碱性氧化物的原始吸附性能是与催化性能有关的两个关键因素。负载在La 2 O 2 CO 3 -R上的Cu具有较高的Cuδ +种类含量和对CO 2的强吸附性导致优异的催化活性。DRIFTS研究表明,在界面区域产生协同碱性位点可以通过适度和选择性地稳定甲醇合成中间体来提高Cu基甲醇催化剂的内在活性。这项工作为碱性氧化物负载的铜催化剂上的CO 2活化提供了新的见解,而铜与镧氧化物之间金属-载体相互作用的鉴定对于稳定铜基催化剂的合理设计是有益的。










































 京公网安备 11010802027423号
京公网安备 11010802027423号