当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Implication of iron nitride species to enhance the catalytic activity and stability of carbon nanotubes supported Fe catalysts for carbon-free hydrogen production via low-temperature ammonia decomposition
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7cy02270k Hui Zhang 1, 2, 3, 4, 5 , Qinmei Gong 1, 2, 3, 4, 5 , Shan Ren 1, 2, 3, 4, 5 , Mahmood Ali Arshid 6, 7, 8 , Wei Chu 5, 9, 10, 11 , Chen Chen 5, 12, 13, 14
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7cy02270k Hui Zhang 1, 2, 3, 4, 5 , Qinmei Gong 1, 2, 3, 4, 5 , Shan Ren 1, 2, 3, 4, 5 , Mahmood Ali Arshid 6, 7, 8 , Wei Chu 5, 9, 10, 11 , Chen Chen 5, 12, 13, 14
Affiliation
|
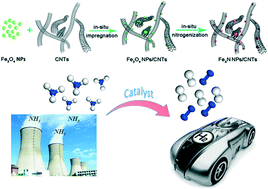
This study was aimed to boost the catalytic ability of carbon nanotubes (CNTs) supported Fe-based catalysts, prepared by using wet-impregnation and followed by nitrogenization, for carbon-free hydrogen production from NH3 decomposition at a low temperature. The nitrogenization temperature and iron loading had significant effects on the size of the well-dispersed Fe2N crystallites. The Fe3O4/CNTs catalysts at a higher nitrogenation temperature under NH3 flow with a suitable Fe content led to the formation of the stable and uniformly distributed Fe2N species, which played an active role in the enhanced catalytic ability of the Fe3O4/CNTs catalysts. However, the nitrogenization of the Fe3O4/CNTs catalyst under either H2 or Ar led to the formation of the Fe4N and Fe2N species. In the presence of the Fe4N phases, the Fe3O4/CNTs catalyst exhibited an enhanced catalytic activity. In brief, the collaborative interaction of the active site Fe2N and carbon nanotubes in the Fe2N/CNTs catalysts resulted in a significant increase in the catalytic activity and durability up to 40 h. The effective control of the density of the active sites Fe2N and the synergism between the carrier and the crystallite composition of iron nitrides are the key aspects for the efficient design of the transition nitride catalysts for carbon-free hydrogen production via ammonia decomposition.
中文翻译:

氮化铁物质对提高碳纳米管负载的Fe催化剂的活性和稳定性的暗示意义在于通过低温氨分解 生产无碳氢的Fe催化剂
这项研究旨在提高碳纳米管(CNTs)负载的铁基催化剂的催化能力,该催化剂通过湿法浸渍然后进行硝化来制备,用于低温下NH 3分解产生的无碳氢。氮化温度和铁负载量对均匀分散的Fe 2 N晶粒的尺寸具有显着影响。Fe含量合适的NH 3流下,在较高的硝化温度下,Fe 3 O 4 / CNTs催化剂导致形成稳定且分布均匀的Fe 2 N物种,对提高Fe的催化能力起积极作用3 Ò 4/ CNTs催化剂。然而,在H 2或Ar下Fe 3 O 4 / CNTs催化剂的氮化导致Fe 4 N和Fe 2 N物质的形成。在Fe 4 N相的存在下,Fe 3 O 4 / CNTs催化剂表现出增强的催化活性。简而言之,Fe 2 N / CNTs催化剂中活性位点Fe 2 N和碳纳米管的协同相互作用导致了长达40小时的催化活性和耐久性的显着提高。有效控制活性位Fe 2的密度N和氮化铁的载体与微晶组成之间的协同作用是高效设计用于通过氨分解生产无碳氢的过渡氮化物催化剂的关键方面。
更新日期:2018-01-04
中文翻译:

氮化铁物质对提高碳纳米管负载的Fe催化剂的活性和稳定性的暗示意义在于通过低温氨分解 生产无碳氢的Fe催化剂
这项研究旨在提高碳纳米管(CNTs)负载的铁基催化剂的催化能力,该催化剂通过湿法浸渍然后进行硝化来制备,用于低温下NH 3分解产生的无碳氢。氮化温度和铁负载量对均匀分散的Fe 2 N晶粒的尺寸具有显着影响。Fe含量合适的NH 3流下,在较高的硝化温度下,Fe 3 O 4 / CNTs催化剂导致形成稳定且分布均匀的Fe 2 N物种,对提高Fe的催化能力起积极作用3 Ò 4/ CNTs催化剂。然而,在H 2或Ar下Fe 3 O 4 / CNTs催化剂的氮化导致Fe 4 N和Fe 2 N物质的形成。在Fe 4 N相的存在下,Fe 3 O 4 / CNTs催化剂表现出增强的催化活性。简而言之,Fe 2 N / CNTs催化剂中活性位点Fe 2 N和碳纳米管的协同相互作用导致了长达40小时的催化活性和耐久性的显着提高。有效控制活性位Fe 2的密度N和氮化铁的载体与微晶组成之间的协同作用是高效设计用于通过氨分解生产无碳氢的过渡氮化物催化剂的关键方面。



























 京公网安备 11010802027423号
京公网安备 11010802027423号