当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Base-promoted hydrolytic dehydrogenation of ammonia borane catalyzed by noble-metal-free nanoparticles†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7cy02365k Qilu Yao 1, 2, 3, 4, 5 , Kun Yang 1, 2, 3, 4, 5 , Xiaoling Hong 1, 2, 3, 4, 5 , Xiangshu Chen 1, 2, 3, 4, 5 , Zhang-Hui Lu 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7cy02365k Qilu Yao 1, 2, 3, 4, 5 , Kun Yang 1, 2, 3, 4, 5 , Xiaoling Hong 1, 2, 3, 4, 5 , Xiangshu Chen 1, 2, 3, 4, 5 , Zhang-Hui Lu 1, 2, 3, 4, 5
Affiliation
|
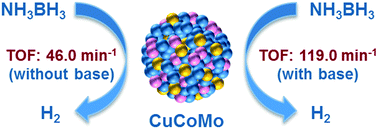
Hydrogen is a potentially ideal alternative energy source for both environmental and economic reasons. The development of sustainable, efficient, and economical catalysts towards hydrogen generation from hydrogen storage materials (e.g., ammonia borane, AB) has been one of the active and challenging research areas. In this work, noble-metal-free CuCoMo nanoparticles (NPs) without any surfactant or support have been prepared via a facile co-reduction method at room temperature and used as highly efficient catalysts for hydrogen generation from an aqueous AB solution. Compared to their monometallic and bimetallic counterparts, the obtained trimetallic Cu0.72Co0.18Mo0.1 NPs exhibit the highest catalytic activity for the hydrolysis of AB with a total turnover frequency (TOF) value of 46.0 min−1 at room temperature under ambient atmosphere. The Cu0.72Co0.18Mo0.1 NPs only needed 0.63 min to completely hydrolyse AB in the presence of a basic solution (NaOH, 1 M) with a TOF value as high as 119.0 min−1 at room temperature, which is among the highest values of all the noble-metal-free catalysts ever reported for the same reaction, wherein NaOH plays an important role in improving the catalytic activity. In addition, it is found that the hydrogen generation rate can be improved by adding a base (e.g., NaOH, KOH, etc.) into the reaction solution, while the presence of NH4+ species (e.g., NH3·H2O, NH4Cl, etc.) is unfavorable for the hydrolysis of AB.
中文翻译:

无贵金属纳米粒子催化的碱促进氨硼烷的水解脱氢†
出于环境和经济原因,氢气是潜在的理想替代能源。对于从储氢材料(例如,氨硼烷,AB)产生氢的可持续,高效和经济的催化剂的开发一直是活跃且具有挑战性的研究领域之一。在这项工作中,已通过一种简便的共还原方法在室温下制备了不含任何表面活性剂或载体的不含贵金属的CuCoMo纳米颗粒(NPs),并将其用作从AB水溶液生成氢的高效催化剂。与单金属和双金属对应物相比,获得的三金属Cu 0.72 Co 0.18 Mo 0.1NP在室温下在室温下对AB的水解表现出最高的催化活性,总周转频率(TOF)值为46.0 min -1。Cu 0.72 Co 0.18 Mo 0.1 NPs仅需0.63分钟即可在室温下TOF值高达119.0 min -1的碱性溶液(NaOH,1 M)存在下完全水解AB ,这是最高值对于相同的反应,有史以来报道过的所有无贵金属催化剂中,NaOH在改善催化活性方面起着重要作用。此外,发现可以通过添加碱(例如,NaOH,KOH等)来提高氢的产生速率。NH 4 +物质(例如NH 3 ·H 2 O,NH 4 Cl等)的存在不利于AB的水解。
更新日期:2018-01-04
中文翻译:

无贵金属纳米粒子催化的碱促进氨硼烷的水解脱氢†
出于环境和经济原因,氢气是潜在的理想替代能源。对于从储氢材料(例如,氨硼烷,AB)产生氢的可持续,高效和经济的催化剂的开发一直是活跃且具有挑战性的研究领域之一。在这项工作中,已通过一种简便的共还原方法在室温下制备了不含任何表面活性剂或载体的不含贵金属的CuCoMo纳米颗粒(NPs),并将其用作从AB水溶液生成氢的高效催化剂。与单金属和双金属对应物相比,获得的三金属Cu 0.72 Co 0.18 Mo 0.1NP在室温下在室温下对AB的水解表现出最高的催化活性,总周转频率(TOF)值为46.0 min -1。Cu 0.72 Co 0.18 Mo 0.1 NPs仅需0.63分钟即可在室温下TOF值高达119.0 min -1的碱性溶液(NaOH,1 M)存在下完全水解AB ,这是最高值对于相同的反应,有史以来报道过的所有无贵金属催化剂中,NaOH在改善催化活性方面起着重要作用。此外,发现可以通过添加碱(例如,NaOH,KOH等)来提高氢的产生速率。NH 4 +物质(例如NH 3 ·H 2 O,NH 4 Cl等)的存在不利于AB的水解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号