当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Redox-active ligand controlled selectivity of vanadium oxidation on Au(100)†
Chemical Science ( IF 8.4 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7sc04752e Christopher D Tempas 1 , Tobias W Morris 1 , David L Wisman 1, 2 , Duy Le 3 , Naseem U Din 3 , Christopher G Williams 1 , Miao Wang 4 , Alexander V Polezhaev 1 , Talat S Rahman 3, 5 , Kenneth G Caulton 1 , Steven L Tait 1, 4
Chemical Science ( IF 8.4 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7sc04752e Christopher D Tempas 1 , Tobias W Morris 1 , David L Wisman 1, 2 , Duy Le 3 , Naseem U Din 3 , Christopher G Williams 1 , Miao Wang 4 , Alexander V Polezhaev 1 , Talat S Rahman 3, 5 , Kenneth G Caulton 1 , Steven L Tait 1, 4
Affiliation
|
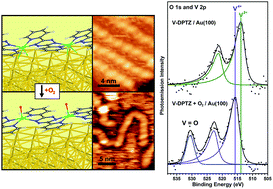
Metal–organic coordination networks at surfaces, formed by on-surface redox assembly, are of interest for designing specific and selective chemical function at surfaces for heterogeneous catalysts and other applications. The chemical reactivity of single-site transition metals in on-surface coordination networks, which is essential to these applications, has not previously been fully characterized. Here, we demonstrate with a surface-supported, single-site V system that not only are these sites active toward dioxygen activation, but the products of that reaction show much higher selectivity than traditional vanadium nanoparticles, leading to only one V-oxo product. We have studied the chemical reactivity of one-dimensional metal–organic vanadium – 3,6-di(2-pyridyl)-1,2,4,5-tetrazine (DPTZ) chains with O2. The electron-rich chains self-assemble through an on-surface redox process on the Au(100) surface and are characterized by X-ray photoelectron spectroscopy, scanning tunneling microscopy, high-resolution electron energy loss spectroscopy, and density functional theory. Reaction of V-DPTZ chains with O2 causes an increase in V oxidation state from VII to VIV, resulting in a single strongly bonded (DPTZ2−)VIVO product and spillover of O to the Au surface. DFT calculations confirm these products and also suggest new candidate intermediate states, providing mechanistic insight into this on-surface reaction. In contrast, the oxidation of ligand-free V is less complete and results in multiple oxygen-bound products. This demonstrates the high chemical selectivity of single-site metal centers in metal–ligand complexes at surfaces compared to metal nanoislands.
中文翻译:

氧化还原活性配体控制 Au(100) 上钒氧化的选择性†
由表面氧化还原组装形成的表面金属有机配位网络对于设计多相催化剂和其他应用表面的特定和选择性化学功能具有重要意义。表面配位网络中单点过渡金属的化学反应性对于这些应用至关重要,但此前尚未得到充分表征。在这里,我们通过表面支撑的单位点 V 系统证明,这些位点不仅对双氧活化具有活性,而且该反应的产物表现出比传统钒纳米颗粒更高的选择性,仅产生一种 V-氧产物。我们研究了一维金属有机钒-3,6-二(2-吡啶基)-1,2,4,5-四嗪(DPTZ)链与O 2的化学反应性。富电子链通过 Au(100) 表面上的表面氧化还原过程自组装,并通过 X 射线光电子能谱、扫描隧道显微镜、高分辨率电子能量损失谱和密度泛函理论进行表征。V-DPTZ 链与 O 2的反应导致 V 氧化态从 V II增加到V IV,从而产生单个强键合的 (DPTZ 2− )V IV O 产物以及 O 溢出到 Au 表面。DFT 计算证实了这些产物,并提出了新的候选中间态,为这种表面反应提供了机械见解。相反,无配体 V 的氧化不太完全,会产生多种氧结合产物。这表明与金属纳米岛相比,表面金属配体复合物中的单点金属中心具有高化学选择性。
更新日期:2018-01-04
中文翻译:

氧化还原活性配体控制 Au(100) 上钒氧化的选择性†
由表面氧化还原组装形成的表面金属有机配位网络对于设计多相催化剂和其他应用表面的特定和选择性化学功能具有重要意义。表面配位网络中单点过渡金属的化学反应性对于这些应用至关重要,但此前尚未得到充分表征。在这里,我们通过表面支撑的单位点 V 系统证明,这些位点不仅对双氧活化具有活性,而且该反应的产物表现出比传统钒纳米颗粒更高的选择性,仅产生一种 V-氧产物。我们研究了一维金属有机钒-3,6-二(2-吡啶基)-1,2,4,5-四嗪(DPTZ)链与O 2的化学反应性。富电子链通过 Au(100) 表面上的表面氧化还原过程自组装,并通过 X 射线光电子能谱、扫描隧道显微镜、高分辨率电子能量损失谱和密度泛函理论进行表征。V-DPTZ 链与 O 2的反应导致 V 氧化态从 V II增加到V IV,从而产生单个强键合的 (DPTZ 2− )V IV O 产物以及 O 溢出到 Au 表面。DFT 计算证实了这些产物,并提出了新的候选中间态,为这种表面反应提供了机械见解。相反,无配体 V 的氧化不太完全,会产生多种氧结合产物。这表明与金属纳米岛相比,表面金属配体复合物中的单点金属中心具有高化学选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号