当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Solubilization of Class B Radical S-Adenosylmethionine Methylases by Improved Cobalamin Uptake in Escherichia coli
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.biochem.7b01205 Nicholas D. Lanz , Anthony J. Blaszczyk , Erin L. McCarthy , Bo Wang , Roy X. Wang , Brianne S. Jones , Squire J. Booker
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.biochem.7b01205 Nicholas D. Lanz , Anthony J. Blaszczyk , Erin L. McCarthy , Bo Wang , Roy X. Wang , Brianne S. Jones , Squire J. Booker
|
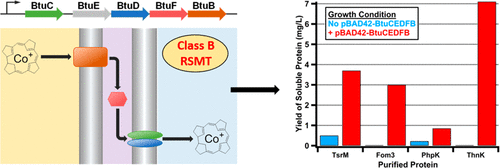
The methylation of unactivated carbon and phosphorus centers is a burgeoning area of biological chemistry, especially given that such reactions constitute key steps in the biosynthesis of numerous enzyme cofactors, antibiotics, and other natural products of clinical value. These kinetically challenging reactions are catalyzed exclusively by enzymes in the radical S-adenosylmethionine (SAM) superfamily and have been grouped into four classes (A–D). Class B radical SAM (RS) methylases require a cobalamin cofactor in addition to the [4Fe-4S] cluster that is characteristic of RS enzymes. However, their poor solubility upon overexpression and their generally poor turnover has hampered detailed in vitro studies of these enzymes. It has been suggested that improper folding, possibly caused by insufficient cobalamin during their overproduction in Escherichia coli, leads to formation of inclusion bodies. Herein, we report our efforts to improve the overproduction of class B RS methylases in a soluble form by engineering a strain of E. coli to take in more cobalamin. We cloned five genes (btuC, btuE, btuD, btuF, and btuB) that encode proteins that are responsible for cobalamin uptake and transport in E. coli and co-expressed these genes with those that encode TsrM, Fom3, PhpK, and ThnK, four class B RS methylases that suffer from poor solubility during overproduction. This strategy markedly enhances the uptake of cobalamin into the cytoplasm and improves the solubility of the target enzymes significantly.
中文翻译:

通过改善大肠杆菌中钴胺素的吸收来增强B类自由基S-腺苷甲硫氨酸甲基化酶的增溶作用
未激活的碳和磷中心的甲基化是生物化学的新兴领域,尤其是考虑到此类反应是众多酶辅因子,抗生素和其他具有临床价值的天然产物生物合成中的关键步骤。这些动力学上具有挑战性的反应仅由自由基S-腺苷甲硫氨酸(SAM)超家族中的酶催化,并且已分为四类(AD)。B类自由基SAM(RS)甲基化酶除了具有RS酶特征的[4Fe-4S]簇外,还需要钴胺素辅助因子。但是,它们在过度表达时的溶解性差,并且其营业额通常较差,这妨碍了详细的体外试验这些酶的研究。已经提出,不适当的折叠可能是由于在大肠杆菌中过量生产时钴胺素不足引起的,导致包涵体的形成。在本文中,我们报告了通过改造大肠杆菌菌株以吸收更多钴胺素来改善可溶性B类RS甲基化酶过量生产的努力。我们克隆了五个基因(btuC,btuE,btuD,btuF和btuB),这些基因编码负责钴胺素在大肠杆菌中摄取和运输的蛋白质。并将这些基因与编码TsrM,Fom3,PhpK和ThnK的基因共表达,这是四种B类RS甲基化酶,在生产过剩期间溶解性较差。该策略显着提高了钴胺素进入细胞质的吸收,并显着提高了目标酶的溶解度。
更新日期:2018-01-03
中文翻译:

通过改善大肠杆菌中钴胺素的吸收来增强B类自由基S-腺苷甲硫氨酸甲基化酶的增溶作用
未激活的碳和磷中心的甲基化是生物化学的新兴领域,尤其是考虑到此类反应是众多酶辅因子,抗生素和其他具有临床价值的天然产物生物合成中的关键步骤。这些动力学上具有挑战性的反应仅由自由基S-腺苷甲硫氨酸(SAM)超家族中的酶催化,并且已分为四类(AD)。B类自由基SAM(RS)甲基化酶除了具有RS酶特征的[4Fe-4S]簇外,还需要钴胺素辅助因子。但是,它们在过度表达时的溶解性差,并且其营业额通常较差,这妨碍了详细的体外试验这些酶的研究。已经提出,不适当的折叠可能是由于在大肠杆菌中过量生产时钴胺素不足引起的,导致包涵体的形成。在本文中,我们报告了通过改造大肠杆菌菌株以吸收更多钴胺素来改善可溶性B类RS甲基化酶过量生产的努力。我们克隆了五个基因(btuC,btuE,btuD,btuF和btuB),这些基因编码负责钴胺素在大肠杆菌中摄取和运输的蛋白质。并将这些基因与编码TsrM,Fom3,PhpK和ThnK的基因共表达,这是四种B类RS甲基化酶,在生产过剩期间溶解性较差。该策略显着提高了钴胺素进入细胞质的吸收,并显着提高了目标酶的溶解度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号