当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium‐Catalyzed Enantioselective Desymmetrizing Aza‐Wacker Reaction: Development and Application to the Total Synthesis of (−)‐Mesembrane and (+)‐Crinane
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201712521 Xu Bao 1 , Qian Wang 1 , Jieping Zhu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201712521 Xu Bao 1 , Qian Wang 1 , Jieping Zhu 1
Affiliation
|
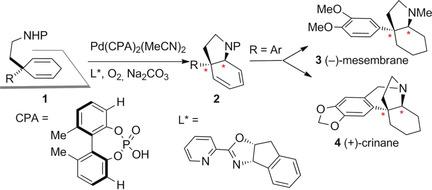
Reported is an unprecedented catalytic enantioselective desymmetrizing aza‐Wacker reaction. In the presence of a catalytic amount of a newly developed Pd(CPA)2(MeCN)2 catalyst (CPA=chiral phosphoric acid), a pyrox ligand, and molecular oxygen, cyclization of properly functionalized prochiral 3,3‐disubstituted cyclohexa‐1,4‐dienes afforded enantioenriched cis‐3a‐substituted tetrahydroindoles in good yields with excellent enantioselectivities. A cooperative effect between the phosphoric acid and the pyrox ligand ensured efficient transformation. This reaction was tailor‐made for Amaryllidaceae and Sceletium alkaloids as illustrated by its application in the development of the concise and divergent total synthesis of (−)‐mesembrane and (+)‐crinane.
中文翻译:

钯催化的对映体选择性对称化Aza-Wacker反应:开发和应用于(-)-膜和(+)-蓖麻烷的全合成
报道了前所未有的催化对映选择性脱对称氮杂-瓦克反应。在催化量的新开发的Pd(CPA)2(MeCN)2催化剂(CPA =手性磷酸),吡咯配体和分子氧的存在下,适当官能化的手性3,3-二取代的环己-1的环化,4-二烯以良好的收率和优异的对映选择性提供了对映体富集的顺式-3a-取代的四氢吲哚。磷酸和吡咯配体之间的协同作用确保了有效的转化。该反应是针对金眼兰科和苦艾碱生物碱量身定制的,如其在开发简明和发散的(-)-膜和(+)-Crinane的全合成反应中的应用所说明的那样。
更新日期:2018-01-16
中文翻译:

钯催化的对映体选择性对称化Aza-Wacker反应:开发和应用于(-)-膜和(+)-蓖麻烷的全合成
报道了前所未有的催化对映选择性脱对称氮杂-瓦克反应。在催化量的新开发的Pd(CPA)2(MeCN)2催化剂(CPA =手性磷酸),吡咯配体和分子氧的存在下,适当官能化的手性3,3-二取代的环己-1的环化,4-二烯以良好的收率和优异的对映选择性提供了对映体富集的顺式-3a-取代的四氢吲哚。磷酸和吡咯配体之间的协同作用确保了有效的转化。该反应是针对金眼兰科和苦艾碱生物碱量身定制的,如其在开发简明和发散的(-)-膜和(+)-Crinane的全合成反应中的应用所说明的那样。











































 京公网安备 11010802027423号
京公网安备 11010802027423号