当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the Fe n+ /Fe (n−1)+ redox potential of Fe phthalocyanines and Fe porphyrins as a reactivity descriptor in the electrochemical oxidation of cysteamine
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.jelechem.2017.12.068 Nataly Silva , Sebastián Calderón , Maritza A. Páez , María P. Oyarzún , Marc T.M. Koper , José H. Zagal
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.jelechem.2017.12.068 Nataly Silva , Sebastián Calderón , Maritza A. Páez , María P. Oyarzún , Marc T.M. Koper , José H. Zagal
|
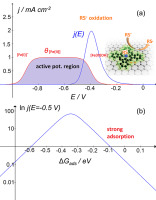
Abstract The Mn +/M(n − 1)+ redox potential of MN4 macrocyclic molecular catalysts is a very good reactivity descriptor for several electrochemical reactions. One important feature about this reactivity descriptor is that it can be determined experimentally under the same conditions of the kinetic measurements in contrast to other descriptors like intermediate binding energies that are estimated from DFT calculations. However a linear correlation between both descriptors seems to exist. Plots of activity as (logj)E at constant E versus the Mn +/M(n − 1)+ redox potential gives volcano correlations. Another important aspect about this parameter is that it is possible to tune the Mn +/M(n − 1)+ redox potential of the MN4 catalyst by manipulating the structure of the macrocyclic complex and tailoring the electron-withdrawing power of the ligands to obtain the maximum activity. In this work we have probed the redox potential as a reactivity descriptor for the oxidation of cysteamine studying a series of substituted Fe phthalocyanines and Fe porphyrins adsorbed on glassy carbon and pyrolytic graphite in alkaline media. As expected the catalytic activity of these FeN4 species varies strongly with the Fe (II)/(I) redox potential of the different Fe phthalocyanines and a plot of activity as (logj)E versus E°Fe(II/I) gives a volcano-shaped correlation so a formal potential value exists for which the highest activity can be achieved demonstrating that the formal potential of the complexes seems to be an universal reactivity descriptor for electrochemical reactions.
中文翻译:

探索 Fe 酞菁和 Fe 卟啉的 Fe n+ /Fe (n−1)+ 氧化还原电位作为半胱胺电化学氧化中的反应性描述符
摘要 MN4 大环分子催化剂的 Mn +/M(n − 1)+ 氧化还原电位是几种电化学反应的一个很好的反应性描述符。关于此反应性描述符的一个重要特征是,它可以在动力学测量的相同条件下通过实验确定,与其他描述符(如从 DFT 计算估计的中间结合能)形成对比。然而,两个描述符之间似乎存在线性相关性。在恒定 E 下作为 (logj)E 的活动图与 Mn +/M(n − 1)+ 氧化还原电位给出了火山相关性。关于该参数的另一个重要方面是可以通过操纵大环配合物的结构和调整配体的吸电子能力来调节 MN4 催化剂的 Mn +/M(n − 1)+ 氧化还原电位以获得最大的活动。在这项工作中,我们探讨了氧化还原电位作为半胱胺氧化的反应性描述符,研究了一系列取代的 Fe 酞菁和 Fe 卟啉吸附在碱性介质中的玻璃碳和热解石墨上。
更新日期:2018-06-01
中文翻译:

探索 Fe 酞菁和 Fe 卟啉的 Fe n+ /Fe (n−1)+ 氧化还原电位作为半胱胺电化学氧化中的反应性描述符
摘要 MN4 大环分子催化剂的 Mn +/M(n − 1)+ 氧化还原电位是几种电化学反应的一个很好的反应性描述符。关于此反应性描述符的一个重要特征是,它可以在动力学测量的相同条件下通过实验确定,与其他描述符(如从 DFT 计算估计的中间结合能)形成对比。然而,两个描述符之间似乎存在线性相关性。在恒定 E 下作为 (logj)E 的活动图与 Mn +/M(n − 1)+ 氧化还原电位给出了火山相关性。关于该参数的另一个重要方面是可以通过操纵大环配合物的结构和调整配体的吸电子能力来调节 MN4 催化剂的 Mn +/M(n − 1)+ 氧化还原电位以获得最大的活动。在这项工作中,我们探讨了氧化还原电位作为半胱胺氧化的反应性描述符,研究了一系列取代的 Fe 酞菁和 Fe 卟啉吸附在碱性介质中的玻璃碳和热解石墨上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号