Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single Molecule Force Spectroscopy Reveals Two-Domain Binding Mode of Pilus-1 Tip Protein RrgA of Streptococcus pneumoniae to Fibronectin
ACS Nano ( IF 15.8 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acsnano.7b07247 Tanja D. Becke 1, 2 , Stefan Ness , Raimund Gürster , Arndt F. Schilling 1, 3 , Anne-Marie di Guilmi 4 , Stefanie Sudhop 2 , Markus Hilleringmann , Hauke Clausen-Schaumann 2
ACS Nano ( IF 15.8 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acsnano.7b07247 Tanja D. Becke 1, 2 , Stefan Ness , Raimund Gürster , Arndt F. Schilling 1, 3 , Anne-Marie di Guilmi 4 , Stefanie Sudhop 2 , Markus Hilleringmann , Hauke Clausen-Schaumann 2
Affiliation
|
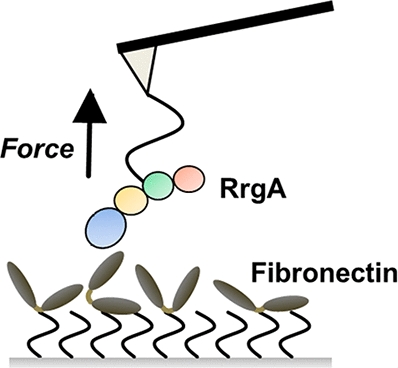
For host cell adhesion and invasion, surface piliation procures benefits for bacteria. A detailed investigation of how pili adhere to host cells is therefore a key aspect in understanding their role during infection. Streptococcus pneumoniae TIGR 4, a clinical relevant serotype 4 strain, is capable of expressing pilus-1 with terminal RrgA, an adhesin interacting with host extracellular matrix (ECM) proteins. We used single molecule force spectroscopy to investigate the binding of full-length RrgA and single RrgA domains to fibronectin. Our results show that full-length RrgA and its terminal domains D3 and D4 bind to fibronectin with forces of 51.6 (full length), 52.8 (D3), and 46.2 pN (D4) at force-loading rates of around 1500 pN/s. Selective saturation of D3 and D4 binding sites on fibronectin showed that both domains can interact simultaneously with fibronectin, revealing a two-domain binding mechanism for the pilus-1 tip protein. The high off rates and the corresponding short lifetime of the RrgA Fn bond (τ = 0.26 s) may enable piliated pneumococci to form and maintain a transient contact to fibronectin-containing host surfaces and thus to efficiently scan the surface for specific receptors promoting host cell adhesion and invasion. These molecular properties could be essential for S. pneumoniae pili to mediate initial contact to the host cells and—shared with other piliated Gram-positive bacteria—favor host invasion.
中文翻译:

单分子力谱揭示肺炎链球菌的Pilus-1尖端蛋白RrgA与纤连蛋白的两个域结合模式。
对于宿主细胞的粘附和侵袭,表面起毛为细菌带来了好处。因此,对菌毛如何附着于宿主细胞的详细研究是了解它们在感染过程中的作用的关键方面。肺炎链球菌TIGR 4是一种与临床相关的血清型4菌株,能够表达具有末端RrgA(与宿主细胞外基质(ECM)蛋白质相互作用的粘附素)的pilus-1。我们使用单分子力谱研究全长RrgA和单个RrgA域与纤连蛋白的结合。我们的结果表明,全长RrgA及其末端结构域D3和D4在大约1500 pN / s的力负载速率下以51.6(全长),52.8(D3)和46.2pN(D4)的力与纤连蛋白结合。纤连蛋白上D3和D4结合位点的选择性饱和表明,两个结构域都可以与纤连蛋白同时相互作用,从而揭示了菌毛1尖端蛋白的两个域的结合机制。RrgA Fn键的高关闭率和相应的短寿命(τ= 0。26 s)可使纤毛状肺炎球菌形成并维持与含纤连蛋白的宿主表面的短暂接触,从而有效地扫描表面以寻找促进宿主细胞粘附和侵袭的特定受体。这些分子特性对于肺炎链球菌菌毛介导与宿主细胞的初始接触,并与其他纤毛革兰氏阳性细菌共享,有利于宿主入侵。
更新日期:2018-01-03
中文翻译:

单分子力谱揭示肺炎链球菌的Pilus-1尖端蛋白RrgA与纤连蛋白的两个域结合模式。
对于宿主细胞的粘附和侵袭,表面起毛为细菌带来了好处。因此,对菌毛如何附着于宿主细胞的详细研究是了解它们在感染过程中的作用的关键方面。肺炎链球菌TIGR 4是一种与临床相关的血清型4菌株,能够表达具有末端RrgA(与宿主细胞外基质(ECM)蛋白质相互作用的粘附素)的pilus-1。我们使用单分子力谱研究全长RrgA和单个RrgA域与纤连蛋白的结合。我们的结果表明,全长RrgA及其末端结构域D3和D4在大约1500 pN / s的力负载速率下以51.6(全长),52.8(D3)和46.2pN(D4)的力与纤连蛋白结合。纤连蛋白上D3和D4结合位点的选择性饱和表明,两个结构域都可以与纤连蛋白同时相互作用,从而揭示了菌毛1尖端蛋白的两个域的结合机制。RrgA Fn键的高关闭率和相应的短寿命(τ= 0。26 s)可使纤毛状肺炎球菌形成并维持与含纤连蛋白的宿主表面的短暂接触,从而有效地扫描表面以寻找促进宿主细胞粘附和侵袭的特定受体。这些分子特性对于肺炎链球菌菌毛介导与宿主细胞的初始接触,并与其他纤毛革兰氏阳性细菌共享,有利于宿主入侵。











































 京公网安备 11010802027423号
京公网安备 11010802027423号