当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insights into Hydride Bonding, Dynamics, and Migration in La2LiHO3 Oxyhydride
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jpclett.7b03098 Øystein S. Fjellvåg 1 , Jeff Armstrong 2 , Ponniah Vajeeston 1 , Anja O. Sjåstad 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jpclett.7b03098 Øystein S. Fjellvåg 1 , Jeff Armstrong 2 , Ponniah Vajeeston 1 , Anja O. Sjåstad 1
Affiliation
|
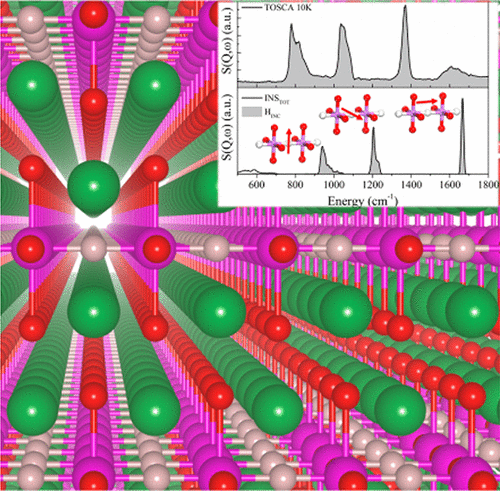
Hydride anion-conducting oxyhydrides have recently emerged as a brand new class of ionic conductors. Here we shed a first light onto their local vibrations, bonding mechanisms, and anion migration properties using the powerful combination of high-resolution inelastic neutron scattering and a set of rigorously experimentally validated density functional theory calculations. By means of charge-density analysis we establish the bonding to be strongly anisotropic; ionic in the perovskite layer and covalent in the rock salt layer. Climbing nudged elastic band calculations allow us to predict the hydride migration paths, which crucially we are able to link to the observed exotic ionic–covalent hybrid bonding nature. In particular, hydride migration in the rock salt layer is seen to be greatly hindered by the presence of covalent bonding, forcing in-plane hydride migration in the perovskite layer to be the dominant transport mechanism. On the basis of this microscopic insight into the transport and bonding, we are able to propose future candidates for materials that are likely to show enhanced hydride conductivity.
中文翻译:

La 2 LiHO 3氧化物中氢化物键合,动力学和迁移的新见解
氢化物负离子传导性的氢氧根已作为一种崭新的离子导体类出现了。在这里,我们使用高分辨率非弹性中子散射和一组经过严格实验验证的密度泛函理论计算的强大组合,首次揭示了它们的局部振动,键合机理和阴离子迁移特性。通过电荷密度分析,我们确定键具有强各向异性。钙钛矿层中的离子和岩盐层中的共价离子。爬升微带计算可以使我们预测氢化物的迁移路径,至关重要的是,我们能够将其与观察到的奇异的离子-共价杂化键结合起来。特别是,发现共价键的存在极大地阻碍了氢化物在盐岩层中的迁移,迫使钙钛矿层中的平面氢化物迁移成为主要的传输机制。基于对传输和键合的微观洞察力,我们能够为可能显示出增强的氢化物导电性的材料提出未来的候选材料。
更新日期:2018-01-08
中文翻译:

La 2 LiHO 3氧化物中氢化物键合,动力学和迁移的新见解
氢化物负离子传导性的氢氧根已作为一种崭新的离子导体类出现了。在这里,我们使用高分辨率非弹性中子散射和一组经过严格实验验证的密度泛函理论计算的强大组合,首次揭示了它们的局部振动,键合机理和阴离子迁移特性。通过电荷密度分析,我们确定键具有强各向异性。钙钛矿层中的离子和岩盐层中的共价离子。爬升微带计算可以使我们预测氢化物的迁移路径,至关重要的是,我们能够将其与观察到的奇异的离子-共价杂化键结合起来。特别是,发现共价键的存在极大地阻碍了氢化物在盐岩层中的迁移,迫使钙钛矿层中的平面氢化物迁移成为主要的传输机制。基于对传输和键合的微观洞察力,我们能够为可能显示出增强的氢化物导电性的材料提出未来的候选材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号