当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Periodic Trends from Metal Substitution in Bimetallic Mo-Based Phosphides for Hydrodeoxygenation and Hydrogenation Reactions
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.jpcc.7b09363 Yolanda Bonita 1 , Jason C. Hicks 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.jpcc.7b09363 Yolanda Bonita 1 , Jason C. Hicks 1
Affiliation
|
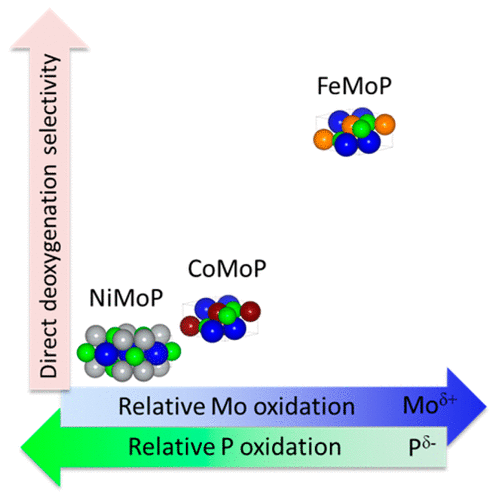
Bimetallic phosphides are promising materials for biomass valorization, yet many metal combinations are understudied as catalysts and require further analysis to realize their superior properties. Herein, we provide the synthesis, characterization, and catalytic performance of a variety of period 4 and 5 solid solutions of molybdenum-based bimetallic phosphides (MMoP, M = Fe, Co, Ni, Ru). From the results, the charge sharing between the metals and phosphorus control the relative oxidation of Mo and reduction of P in the lattice, which were both indirectly observed in binding energy shifts in X-ray photoelectron spectroscopy (XPS) and absorption energy shifts in X-ray absorption near-edge spectroscopy (XANES). For MMoP (M = Fe, Co, Ni), the more oxidized the Mo in the bimetallic phosphide, the higher the selectivity to benzene from phenol via direct deoxygenation at 400 °C and 750 psig. This phenomenon was observed in the bimetallic materials synthesized across period 4, where aromatic selectivity and degree of Mo oxidation both decreased in the following order FeMoP ≫ CoMoP > NiMoP. Alternatively, in the case of MMoP (M = Fe, Ru), the P in RuMoP is more oxidized compared to that in FeMoP, and the selectivity toward the hydrogenation pathway increased due to the interaction between the aromatic rings and the P species on the surface. For RuMoP and NiMoP, cyclohexanol was selectively produced from phenol with >99% selectivity when the reaction temperature was lowered to 125 °C at 750 psig, whereas FeMoP and CoMoP were not active under these conditions. Last, complete deoxygenation of phenol to benzene, cyclohexane, and cyclohexene was accomplished using mixtures of RuMoP and FeMoP in flow and batch experiments. These results highlight the versatility and wide applicability of transition metal phosphides for biomass conversions.
中文翻译:

钼基双金属加氢脱氧和加氢反应中金属取代的周期性趋势
双金属磷化物是用于生物量增值的有前途的材料,但是许多金属组合物仍被研究为催化剂,需要进一步分析以实现其优越的性能。本文中,我们提供了钼基双金属磷化物(M MoP,M = Fe,Co,Ni,Ru)的各种周期4和5固溶体的合成,表征和催化性能。从结果来看,金属和磷之间的电荷共享控制了晶格中Mo的相对氧化和P的还原,这在X射线光电子能谱(XPS)的结合能移动和X的吸收能移动中都是间接观察到的射线吸收近边缘光谱仪(XANES)。对于MMoP(M = Fe,Co,Ni),双金属磷化物中的Mo氧化越多,在400°C和750 psig下通过直接脱氧从苯酚对苯的选择性越高。在整个周期4合成的双金属材料中观察到了这种现象,其中芳族选择性和Mo氧化度均以FeMoP≫ CoMoP> NiMoP的顺序降低。或者,在M的情况下MoP(M = Fe,Ru),RuMoP中的P与FeMoP中的P相比被氧化,并且由于芳环与表面P物种之间的相互作用,对氢化途径的选择性增加。对于RuMoP和NiMoP,当在750 psig下将反应温度降至125°C时,苯酚选择性地以> 99%的选择性生成环己醇,而FeMoP和CoMoP在这些条件下没有活性。最后,在流动和分批实验中,使用RuMoP和FeMoP的混合物将苯酚完全脱氧为苯,环己烷和环己烯。这些结果突出了过渡金属磷化物用于生物质转化的多功能性和广泛的适用性。
更新日期:2018-01-03
中文翻译:

钼基双金属加氢脱氧和加氢反应中金属取代的周期性趋势
双金属磷化物是用于生物量增值的有前途的材料,但是许多金属组合物仍被研究为催化剂,需要进一步分析以实现其优越的性能。本文中,我们提供了钼基双金属磷化物(M MoP,M = Fe,Co,Ni,Ru)的各种周期4和5固溶体的合成,表征和催化性能。从结果来看,金属和磷之间的电荷共享控制了晶格中Mo的相对氧化和P的还原,这在X射线光电子能谱(XPS)的结合能移动和X的吸收能移动中都是间接观察到的射线吸收近边缘光谱仪(XANES)。对于MMoP(M = Fe,Co,Ni),双金属磷化物中的Mo氧化越多,在400°C和750 psig下通过直接脱氧从苯酚对苯的选择性越高。在整个周期4合成的双金属材料中观察到了这种现象,其中芳族选择性和Mo氧化度均以FeMoP≫ CoMoP> NiMoP的顺序降低。或者,在M的情况下MoP(M = Fe,Ru),RuMoP中的P与FeMoP中的P相比被氧化,并且由于芳环与表面P物种之间的相互作用,对氢化途径的选择性增加。对于RuMoP和NiMoP,当在750 psig下将反应温度降至125°C时,苯酚选择性地以> 99%的选择性生成环己醇,而FeMoP和CoMoP在这些条件下没有活性。最后,在流动和分批实验中,使用RuMoP和FeMoP的混合物将苯酚完全脱氧为苯,环己烷和环己烯。这些结果突出了过渡金属磷化物用于生物质转化的多功能性和广泛的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号