Organic Electronics ( IF 2.7 ) Pub Date : 2018-01-03 , DOI: 10.1016/j.orgel.2017.12.052 Jie Min , Yuriy N. Luponosov , Dmitry A. Khanin , Petr V. Dmitryakov , Evgeniya A. Svidchenko , Svetlana M. Peregudova , Linda Grodd , Souren Grigorian , Sergei N. Chvalun , Sergei A. Ponomarenko , Christoph J. Brabec
|
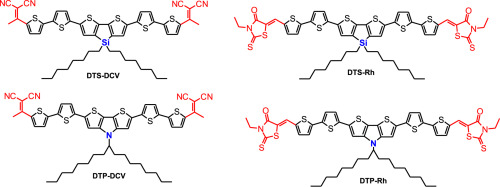
The synthesis of a series of novel A-π-D-π-A oligomers bearing either electron-donating dithieno[3,2-b:2′,3′-d]silole (DTS) or dithieno[3,2-b:2′,3′-d]pyrrole (DTP) units linked through a bithiophene π-bridge with the electron-withdrawing methyldicyanovinyl (DCV) or N-ethylrhodanine (Rh) groups is described. In order to evaluate the effects of different donor-acceptor combinations on various physical properties of the oligomers they are comprehensively studied by UV–Vis spectroscopy, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and cyclic voltammetry (CV). Replacing the DTS by DTP as well as the DCV by Rh leads to increasing of HOMO energy levels and slight decreasing of LUMO energy levels, respectively. The DTP-based oligomers have a red shift in absorption spectra and an increased solubility as compared to the DTS-based analogs. Replacing the acceptor DCV unit by Rh also results in significantly higher solubility, but the Rh-based oligomers exhibit weaker intermolecular interactions, poorer optical absorption of sun light as well as lower charge carrier mobility in blends. Altogether, the structural improvement of the DTS-DCV and DTP-DCV blends upon annealing correlates well with the observed photovoltaic performances in organic solar cells. These results give more insight how to fine-tune and predict physical properties and photovoltaic performance of small A-π-D-π-A molecules having different donor-acceptor combinations in their chemical structures and thus providing a molecular design guideline for the next generation of high-performance photovoltaic materials.
中文翻译:

供体单元中桥联原子和受体基团性质对A-π-D-π-A低聚物物理和光伏性质的影响
带有电子给体双噻吩并[3,2- b:2',3'- d ]硅酮(DTS)或双噻吩并[3,2- b ]的一系列新型A-π-D-π-A低聚物的合成:2′,3′- d描述了通过联噻吩π桥与吸电子的甲基二氰基乙烯基(DCV)或N-乙基罗丹宁(Rh)基团连接的]吡咯(DTP)单元。为了评估不同供体-受体组合对低聚物各种物理性质的影响,已通过紫外可见光谱,差示扫描量热法(DSC),热重分析(TGA)和循环伏安法(CV)对其进行了全面研究。用DTP代替DTS以及用Rh代替DCV分别导致HOMO能级增加和LUMO能级稍微降低。与基于DTS的类似物相比,基于DTP的低聚物具有吸收光谱的红移和增加的溶解度。用Rh取代受体DCV单元也会显着提高溶解度,但是Rh基低聚物表现出较弱的分子间相互作用,较弱的太阳光吸收率以及较低的共混物中的载流子迁移率。总而言之,DTS-DCV和DTP-DCV共混物在退火后的结构改进与有机太阳能电池中观察到的光伏性能密切相关。这些结果为如何微调和预测化学结构中具有不同供体-受体组合的小A-π-D-π-A分子的物理性质和光伏性能提供了更多见识,从而为下一代提供了分子设计指南高性能光伏材料。退火后DTS-DCV和DTP-DCV混合物的结构改进与有机太阳能电池中观察到的光伏性能密切相关。这些结果为如何微调和预测化学结构中具有不同供体-受体组合的小A-π-D-π-A分子的物理性质和光伏性能提供了更多见识,从而为下一代提供了分子设计指南高性能光伏材料。DTS-DCV和DTP-DCV共混物在退火后的结构改进与有机太阳能电池中观察到的光伏性能密切相关。这些结果为如何微调和预测化学结构中具有不同供体-受体组合的小A-π-D-π-A分子的物理性质和光伏性能提供了更多见识,从而为下一代提供了分子设计指南高性能光伏材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号