当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isolation, Derivative Synthesis, and Structure–Activity Relationships of Antiparasitic Bromopyrrole Alkaloids from the Marine Sponge Tedania brasiliensis
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00876 Lizbeth L L Parra 1 , Ariane F Bertonha 1, 2 , Ivan R M Severo 1 , Anna C C Aguiar 3 , Guilherme E de Souza 3 , Glaucius Oliva 3 , Rafael V C Guido 3 , Nathalia Grazzia 4 , Tábata R Costa 4 , Danilo C Miguel 4 , Fernanda R Gadelha 4 , Antonio G Ferreira 5 , Eduardo Hajdu 6 , Daniel Romo 2 , Roberto G S Berlinck 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00876 Lizbeth L L Parra 1 , Ariane F Bertonha 1, 2 , Ivan R M Severo 1 , Anna C C Aguiar 3 , Guilherme E de Souza 3 , Glaucius Oliva 3 , Rafael V C Guido 3 , Nathalia Grazzia 4 , Tábata R Costa 4 , Danilo C Miguel 4 , Fernanda R Gadelha 4 , Antonio G Ferreira 5 , Eduardo Hajdu 6 , Daniel Romo 2 , Roberto G S Berlinck 1
Affiliation
|
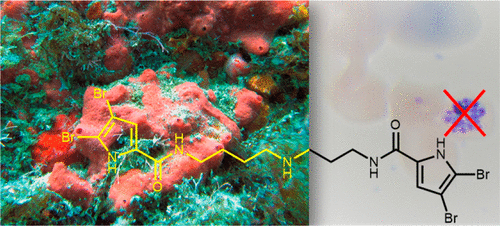
The isolation and identification of a series of new pseudoceratidine (1) derivatives from the sponge Tedania brasiliensis enabled the evaluation of their antiparasitic activity against Plasmodium falciparum, Leishmania (Leishmania) amazonensis, Leishmania (Leishmania) infantum, and Trypanosoma cruzi, the causative agents of malaria, cutaneous leishmaniasis, visceral leishmaniasis, and Chagas disease, respectively. The new 3-debromopseudoceratidine (4), 20-debromopseudoceratidine (5), 4-bromopseudoceratidine (6), 19-bromopseudoceratidine (7), and 4,19-dibromopseudoceratidine (8) are reported. New tedamides A–D (9–12), with an unprecedented 4-bromo-4-methoxy-5-oxo-4,5-dihydro-1H-pyrrole-2-carboxamide moiety, are also described. Compounds 4 and 5, 6 and 7, 9 and 10, and 11 and 12 have been isolated as pairs of inseparable structural isomers differing in their sites of bromination or oxidation. Tedamides 9+10 and 11+12 were obtained as optically active pairs, indicating an enzymatic formation rather than an artifactual origin. N12-Acetylpseudoceratidine (2) and N12-formylpseudoceratidine (3) were obtained by derivatization of pseudoceratidine (1). The antiparasitic activity of pseudoceratidine (1) led us to synthesize 23 derivatives (16, 17, 20, 21, 23, 25, 27–29, 31, 33, 35, 38, 39, 42, 43, 46, 47, 50, and 51) with variations in the polyamine chain and aromatic moiety in sufficient amounts for biological evaluation in antiparasitic assays. The measured antimalarial activity of pseudoceratidine (1) and derivatives 4, 5, 16, 23, 25, 31, and 50 provided an initial SAR evaluation of these compounds as potential leads for antiparasitics against Leishmania amastigotes and against P. falciparum. The results obtained indicate that pseudoceratidine represents a promising scaffold for the development of new antimalarial drugs.
中文翻译:

海洋海绵 Tedania brasiliensis 抗寄生虫溴吡咯生物碱的分离、衍生物合成及构效关系
从海绵Tedania brasiliensis中分离和鉴定了一系列新的假角鲨烷 ( 1 ) 衍生物,从而能够评估它们对恶性疟原虫、亚马逊利什曼原虫、婴儿利什曼原虫和克氏锥虫的抗寄生虫活性。分别是疟疾、皮肤利什曼病、内脏利什曼病和恰加斯病。报道了新的 3-去溴假角鲨烷 ( 4 )、20-去溴假角鲨烷( 5 )、4-溴假角鲨烷 ( 6 )、19-溴假角鲨烷 ( 7 ) 和 4,19-二溴假角鲨烷 ( 8 ) 。还描述了新的 tedamides A–D ( 9 – 12 ),其具有前所未有的 4-bromo-4-methoxy-5-oxo-4,5-dihydro-1 H -pyrrole-2-carboxamide 部分。化合物4和5、6和7、9和10以及11和12已被分离为不可分离的结构异构体对,其溴化或氧化位点不同。Tedamides 9 + 10和11 + 12作为光学活性对获得,表明酶促形成而不是人为起源。将假角鲨烷( 1 )衍生化得到N 12 -乙酰假角鲨烷( 2 )和N 12 -甲酰基假角鲨烷( 3 ) 。假角鲨烷 ( 1 )的抗寄生虫活性使我们合成了 23 个衍生物 ( 16 , 17 , 20 , 21 , 23 , 25 , 27 – 29 , 31 , 33 , 35 , 38 , 39 , 42 , 43 , 46 , 47 , 50 ),和51),多胺链和芳香族部分的变化足以用于抗寄生虫测定中的生物学评估。假西雷替丁 ( 1 ) 及其衍生物4的抗疟活性测定、5、16、23、25、31和50对这些化合物作为抗利什曼原虫无鞭毛体和恶性疟原虫抗寄生虫药的潜在先导化合物进行了初步SAR评估。获得的结果表明,假角鲨烷是开发新型抗疟药物的有前途的支架。
更新日期:2018-01-03
中文翻译:

海洋海绵 Tedania brasiliensis 抗寄生虫溴吡咯生物碱的分离、衍生物合成及构效关系
从海绵Tedania brasiliensis中分离和鉴定了一系列新的假角鲨烷 ( 1 ) 衍生物,从而能够评估它们对恶性疟原虫、亚马逊利什曼原虫、婴儿利什曼原虫和克氏锥虫的抗寄生虫活性。分别是疟疾、皮肤利什曼病、内脏利什曼病和恰加斯病。报道了新的 3-去溴假角鲨烷 ( 4 )、20-去溴假角鲨烷( 5 )、4-溴假角鲨烷 ( 6 )、19-溴假角鲨烷 ( 7 ) 和 4,19-二溴假角鲨烷 ( 8 ) 。还描述了新的 tedamides A–D ( 9 – 12 ),其具有前所未有的 4-bromo-4-methoxy-5-oxo-4,5-dihydro-1 H -pyrrole-2-carboxamide 部分。化合物4和5、6和7、9和10以及11和12已被分离为不可分离的结构异构体对,其溴化或氧化位点不同。Tedamides 9 + 10和11 + 12作为光学活性对获得,表明酶促形成而不是人为起源。将假角鲨烷( 1 )衍生化得到N 12 -乙酰假角鲨烷( 2 )和N 12 -甲酰基假角鲨烷( 3 ) 。假角鲨烷 ( 1 )的抗寄生虫活性使我们合成了 23 个衍生物 ( 16 , 17 , 20 , 21 , 23 , 25 , 27 – 29 , 31 , 33 , 35 , 38 , 39 , 42 , 43 , 46 , 47 , 50 ),和51),多胺链和芳香族部分的变化足以用于抗寄生虫测定中的生物学评估。假西雷替丁 ( 1 ) 及其衍生物4的抗疟活性测定、5、16、23、25、31和50对这些化合物作为抗利什曼原虫无鞭毛体和恶性疟原虫抗寄生虫药的潜在先导化合物进行了初步SAR评估。获得的结果表明,假角鲨烷是开发新型抗疟药物的有前途的支架。







































 京公网安备 11010802027423号
京公网安备 11010802027423号