Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-01-03 , DOI: 10.1016/j.bioorg.2017.12.034 Eva Havránková , Jozef Csöllei , Daniela Vullo , Vladimír Garaj , Pavel Pazdera , Claudiu T. Supuran
|
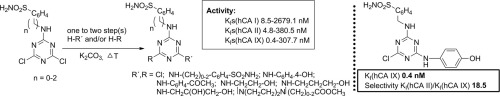
A new series of s-triazine derivatives incorporating sulfanilamide, homosulfanilamide, 4-aminoethyl-benzenesulfonamide and piperazine or aminoalcohol structural motifs is reported. Molecular docking was exploited to select compounds from virtual combinatorial library for synthesis and subsequent biological evaluation. The compounds were prepared by using step by step nucleophilic substitution of chlorine atoms from cyanuric chloride (2,4,6-trichloro-1,3,5-triazine). The compounds were tested as inhibitors of physiologically relevant carbonic anhydrase (CA, EC 4.2.1.1) isoforms. Specifically, against the cytosolic hCA I, II and tumor-associated hCA IX. These compounds show appreciable inhibition. hCA I was inhibited with KIs in the range of 8.5–2679.1 nM, hCA II with KIs in the range of 4.8–380.5 nM and hCA IX with KIs in the range of 0.4–307.7 nM. As other similar derivatives, some of the compounds showed good or excellent selectivity ratios for inhibiting hCA IX over hCA II, of 3.5–18.5. 4-[({4-Chloro-6-[(4-hydroxyphenyl)amino]-1,3,5-triazin-2-yl}amino)methyl] benzene sulfonamide demonstrated subnanomolar affinity for hCA IX (0.4 nM) and selectivity (18.50) over the cytosolic isoforms. This series of compounds may be of interest for the development of new, unconventional anticancer drugs targeting hypoxia-induced CA isoforms such as CA IX.
中文翻译:

新型磺酰胺结合了哌嗪,氨基醇和1,3,5-三嗪结构基序,具有碳酸酐酶I,II和IX抑制作用
据报道,一系列新的β-三嗪衍生物结合了磺胺,高磺胺,4-氨基乙基-苯磺酰胺和哌嗪或氨基醇结构基序。利用分子对接从虚拟组合库中选择化合物进行合成和随后的生物学评估。通过逐步地使用氰尿酰氯(2,4,6-三氯-1,3,5-三嗪)中氯原子的亲核取代来制备化合物。测试了这些化合物作为生理相关的碳酸酐酶(CA,EC 4.2.1.1)同工型的抑制剂。具体而言,针对细胞质hCA I,II和与肿瘤相关的hCA IX。这些化合物显示出明显的抑制作用。hCA I在8.5–2679.1 nM范围内被K I抑制,hCA II被抑制。ķ我S IN 4.8-380.5纳米和HCA IX与范围ķ我S IN 0.4-307.7以下的范围内。与其他类似的衍生物一样,某些化合物在抑制hCA IX方面优于hCA II方面表现出良好或极好的选择性,为3.5-18.5。4-[({4-氯-6-[(4-羟基苯基)氨基] -1,3,5-三嗪-2-基}氨基)甲基]苯磺酰胺显示对hCA IX的亚纳摩尔亲和力(0.4 nM)和选择性(18.50)超过胞质亚型。该系列化合物可能对开发针对缺氧诱导的CA同工型(例如CA IX)的新型非常规抗癌药物感兴趣。











































 京公网安备 11010802027423号
京公网安备 11010802027423号