European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-01-02 , DOI: 10.1016/j.ejmech.2017.12.094 Yun-Kai Shi , Bo Wang , Xiao-Li Shi , Yuan-Di Zhao , Bin Yu , Hong-Min Liu
|
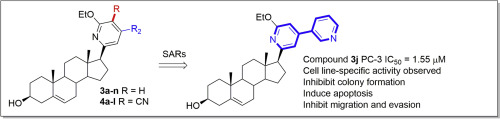
A series of new steroidal pyridines have been synthesized through the based-promoted three-component reaction and preliminarily evaluated for their antiproliferative activity against different types of cancer cell lines. SARs studies showed that the heterocyclic rings attached to the 4-position of the pyridine ring were preferred over the phenyl rings for the activity. Among these compounds, the most potent compound exhibited good growth inhibition against all the tested cancer cells, especially for PC-3 cells with an IC50 value of 1.55 μM. Further mechanistic studies revealed that the most potent compound inhibited colony formation, migration and evasion of PC-3 cells in a concentration-dependent manner as well as induced apoptosis of PC-3 cells possibly through the mitochondria-related apoptotic pathways. Caspase-3/-9 and PARP were activated, finally leading to the apoptosis of PC-3 cells. For the androgen-sensitive (AR+) prostate cancer cell line LNCaP, the most potent compound was less potent than abiraterone with the IC50 value of 8.48 and 3.29 μM, respectively. The most potent compound could be used as a starting point for the development of new steroidal heterocycles with improved anticancer potency and selectivity. The synthesized steroidal pyridines contain the functional -OEt and CN groups, which could be used for further modifications for the construction of the steroid library.
中文翻译:

新的甾体吡啶类化合物作为潜在的抗前列腺癌药物的合成及生物学评价
通过基于基础的三组分反应合成了一系列新的甾族吡啶,并初步评估了它们对不同类型癌细胞系的抗增殖活性。SARs研究表明,与吡啶环相比,连接吡啶环4位的杂环具有更好的活性。在这些化合物中,最有效的化合物对所有测试的癌细胞均表现出良好的生长抑制作用,尤其是对于具有IC 50的PC-3细胞值为1.55μM。进一步的机理研究表明,最有效的化合物可能以浓度依赖的方式抑制PC-3细胞的集落形成,迁移和逃逸,并可能通过线粒体相关的凋亡途径诱导PC-3细胞凋亡。Caspase-3 / -9和PARP被激活,最终导致PC-3细胞凋亡。对于雄激素敏感(AR +)前列腺癌细胞系LNCaP,使用IC 50时,最有效的化合物的效力低于阿比特龙值分别为8.48和3.29μM。最有效的化合物可以用作开发具有改进的抗癌效力和选择性的新型甾族杂环的起点。合成的类固醇吡啶含有功能性-OEt和CN基团,可用于进一步修饰以构建类固醇文库。









































 京公网安备 11010802027423号
京公网安备 11010802027423号