Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-01-02 , DOI: 10.1016/j.bmcl.2018.01.001 Jumpei Morimoto , Yuki Hosono , Shinsuke Sando
|
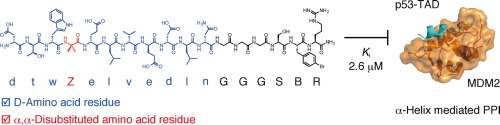
α-Helix-mediated protein–protein interactions (PPIs) are important targets in biological research and drug development. Peptides containing d-amino acid residues are attractive molecules for inhibiting α-helix-mediated PPIs because of their wide surface area and high protease resistance. In this study, a peptide library was constructed using a one-bead one-compound format designed to isolate left-handed α-helical peptides, which are promising molecules as inhibitors of α-helix-mediated PPIs. Screening of the library against an α-helix-mediated PPI between MDM2 and p53 yielded an inhibitor of the PPI. Design and screening of the library, and biochemical and spectroscopic studies of the discovered peptide are presented.
中文翻译:

从一个珠子的一个化合物库中分离出一种含有d-氨基酸残基的肽,该肽可抑制α-螺旋介导的p53-MDM2相互作用
α-螺旋介导的蛋白质-蛋白质相互作用(PPI)是生物学研究和药物开发的重要目标。含有d-氨基酸残基的肽由于其宽的表面积和高的蛋白酶抗性而成为抑制α-螺旋介导的PPI的有吸引力的分子。在这项研究中,使用单珠单化合物格式构建了一个肽库,该格式旨在分离左旋α-螺旋肽,左旋α-螺旋肽是有希望成为α-螺旋介导的PPI抑制剂的分子。针对MDM2和p53之间的α-螺旋介导的PPI筛选文库产生了PPI抑制剂。介绍了文库的设计和筛选,以及发现的肽的生化和光谱学研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号