Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-01-02 , DOI: 10.1016/j.bmc.2017.12.045 Yosuke Ota , Shin Miyamura , Misaho Araki , Yukihiro Itoh , Shusuke Yasuda , Mitsuharu Masuda , Tomoyuki Taniguchi , Yoshihiro Sowa , Toshiyuki Sakai , Kenichiro Itami , Junichiro Yamaguchi , Takayoshi Suzuki
|
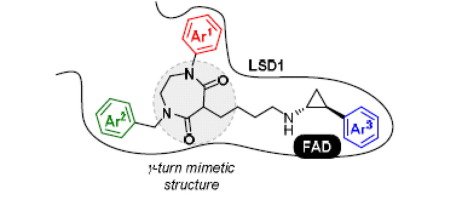
Lysine-specific demethylase 1 (LSD1) is an attractive molecular target for cancer therapy. We have previously reported potent LSD1-selective inhibitors (i.e., NCD18, NCD38, and their analogs) consisting of trans-2-phenylcyclopropylamine (PCPA) or trans-2-arylcyclopropylamine (ACPA) and a lysine moiety that could form a γ-turn structure in the active site of LSD1. Herein we report the design, synthesis and evaluation of γ-turn mimetic compounds for further improvement of LSD1 inhibitory activity and anticancer activity. Among a series of γ-turn mimetic compounds synthesized by a Mitsunobu-reaction-based amination strategy, we identified 1n as a potent and selective LSD1 inhibitor. Compound 1n induced cell cycle arrest and apoptosis through histone methylation in human lung cancer cells. The γ-turn mimetics approach should offer new insights into drug design for LSD1-selective inhibitors.
中文翻译:

作为LSD1选择性抑制剂的γ转模拟物的设计,合成和评估
赖氨酸特异性脱甲基酶1(LSD1)是用于癌症治疗的有吸引力的分子靶标。先前我们已经报道了由反-2-苯基环丙胺(PCPA)或反-2-芳基环丙胺(ACPA)和可能形成γ转角的赖氨酸部分组成的有效LSD1选择性抑制剂(即NCD18,NCD38及其类似物)LSD1活动站点中的结构。在本文中,我们报告了γ-turn模拟化合物的设计,合成和评估,以进一步改善LSD1抑制活性和抗癌活性。在通过Mitsunobu反应为基础的胺化策略合成的一系列γ转模拟化合物中,我们确定1n为有效且选择性的LSD1抑制剂。化合物1n通过组蛋白甲基化诱导人肺癌细胞的细胞周期停滞和凋亡。γ转模拟方法应为LSD1选择性抑制剂的药物设计提供新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号