当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Desymmetrization of Methylenedianilines via Enzyme-Catalyzed Remote Halogenation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-08 , DOI: 10.1021/jacs.7b09573 James T Payne 1 , Paul H Butkovich 1 , Yifan Gu 1 , Kyle N Kunze 1 , Hyun June Park 1 , Duo-Sheng Wang 1 , Jared C Lewis 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-08 , DOI: 10.1021/jacs.7b09573 James T Payne 1 , Paul H Butkovich 1 , Yifan Gu 1 , Kyle N Kunze 1 , Hyun June Park 1 , Duo-Sheng Wang 1 , Jared C Lewis 1
Affiliation
|
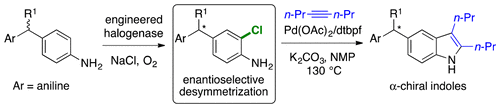
Extensive effort has been devoted to engineering flavin-dependent halogenases (FDHs) with improved stability, expanded substrate scope, and altered regioselectivity. Here, we show that variants of rebeccamycin halogenase (RebH) catalyze enantioselective desymmetrization of methylenedianilines via halogenation of these substrates distal to their pro-stereogenic center. Structure-guided engineering was used to increase the conversion and selectivity of these reactions, and the synthetic utility of the halogenated products was shown via conversion of to a chiral α-substituted indole. These results constitute the first reported examples of asymmetric catalysis by FDHs.
中文翻译:

通过酶催化远程卤化实现亚甲基二苯胺的对映选择性去对称化
人们付出了大量的努力来工程化黄素依赖性卤化酶(FDH),以提高稳定性、扩大底物范围和改变区域选择性。在这里,我们发现瑞贝卡霉素卤化酶(RebH)的变体通过远离其前立体中心的这些底物的卤化来催化亚甲基双苯胺的对映选择性去对称化。结构引导工程用于提高这些反应的转化率和选择性,并且通过将 α-取代吲哚转化为手性α-取代吲哚,显示了卤化产物的合成效用。这些结果构成了 FDH 不对称催化的首次报道实例。
更新日期:2018-01-08
中文翻译:

通过酶催化远程卤化实现亚甲基二苯胺的对映选择性去对称化
人们付出了大量的努力来工程化黄素依赖性卤化酶(FDH),以提高稳定性、扩大底物范围和改变区域选择性。在这里,我们发现瑞贝卡霉素卤化酶(RebH)的变体通过远离其前立体中心的这些底物的卤化来催化亚甲基双苯胺的对映选择性去对称化。结构引导工程用于提高这些反应的转化率和选择性,并且通过将 α-取代吲哚转化为手性α-取代吲哚,显示了卤化产物的合成效用。这些结果构成了 FDH 不对称催化的首次报道实例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号