当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
REDOR NMR Reveals Multiple Conformers for a Protein Kinase C Ligand in a Membrane Environment
ACS Central Science ( IF 12.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acscentsci.7b00475 Hao Yang 1 , Daryl Staveness 2 , Steven M Ryckbosch 2 , Alison D Axtman 2 , Brian A Loy 2 , Alexander B Barnes 1 , Vijay S Pande 2 , Jacob Schaefer 1 , Paul A Wender 2, 3 , Lynette Cegelski 2
ACS Central Science ( IF 12.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acscentsci.7b00475 Hao Yang 1 , Daryl Staveness 2 , Steven M Ryckbosch 2 , Alison D Axtman 2 , Brian A Loy 2 , Alexander B Barnes 1 , Vijay S Pande 2 , Jacob Schaefer 1 , Paul A Wender 2, 3 , Lynette Cegelski 2
Affiliation
|
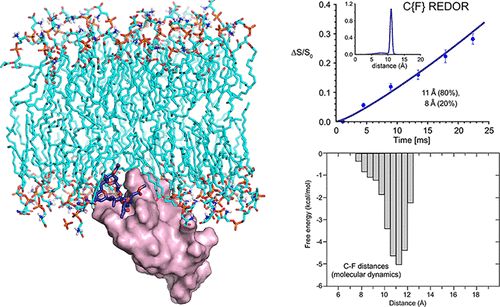
Bryostatin 1 (henceforth bryostatin) is in clinical trials for the treatment of Alzheimer’s disease and for HIV/AIDS eradication. It is also a preclinical lead for cancer immunotherapy and other therapeutic indications. Yet nothing is known about the conformation of bryostatin bound to its protein kinase C (PKC) target in a membrane microenvironment. As a result, efforts to design more efficacious, better tolerated, or more synthetically accessible ligands have been limited to structures that do not include PKC or membrane effects known to influence PKC–ligand binding. This problem extends more generally to many membrane-associated proteins in the human proteome. Here, we use rotational-echo double-resonance (REDOR) solid-state NMR to determine the conformations of PKC modulators bound to the PKCδ-C1b domain in the presence of phospholipid vesicles. The conformationally limited PKC modulator phorbol diacetate (PDAc) is used as an initial test substrate. While unanticipated partitioning of PDAc between an immobilized protein-bound state and a mobile state in the phospholipid assembly was observed, a single conformation in the bound state was identified. In striking contrast, a bryostatin analogue (bryolog) was found to exist exclusively in a protein-bound state, but adopts a distribution of conformations as defined by three independent distance measurements. The detection of multiple PKCδ-C1b-bound bryolog conformers in a functionally relevant phospholipid complex reveals the inherent dynamic nature of cellular systems that is not captured with single-conformation static structures. These results indicate that binding, selectivity, and function of PKC modulators, as well as the design of new modulators, are best addressed using a dynamic multistate model, an analysis potentially applicable to other membrane-associated proteins.
中文翻译:

REDOR NMR 揭示了膜环境中蛋白激酶 C 配体的多个构象异构体
苔藓抑素 1(以下简称苔藓抑素)正处于治疗阿尔茨海默病和根除艾滋病毒/艾滋病的临床试验中。它也是癌症免疫疗法和其他治疗适应症的临床前先导。然而,对于膜微环境中苔藓抑素与其蛋白激酶 C (PKC) 靶标结合的构象一无所知。因此,设计更有效、耐受性更好或更容易合成的配体的努力仅限于不包括 PKC 或已知影响 PKC 配体结合的膜效应的结构。这个问题更普遍地延伸到人类蛋白质组中的许多膜相关蛋白。在这里,我们使用旋转回波双共振 (REDOR) 固态 NMR 来确定在磷脂囊泡存在的情况下与 PKCδ-C1b 结构域结合的 PKC 调节剂的构象。构象限制的 PKC 调节剂佛波醇二乙酸酯 (PDAc) 用作初始测试底物。虽然观察到 PDAc 在磷脂组件中的固定蛋白结合状态和移动状态之间发生意外分配,但鉴定出了结合状态的单一构象。与此形成鲜明对比的是,苔藓抑素类似物(bryolog)被发现仅以蛋白质结合状态存在,但采用由三个独立距离测量定义的构象分布。在功能相关的磷脂复合物中检测多个 PKCδ-C1b 结合的苔藓同构异构体揭示了细胞系统固有的动态性质,而单构象静态结构无法捕获这种性质。 这些结果表明,PKC 调节剂的结合、选择性和功能以及新调节剂的设计最好使用动态多态模型来解决,该分析可能适用于其他膜相关蛋白。
更新日期:2018-01-02
中文翻译:

REDOR NMR 揭示了膜环境中蛋白激酶 C 配体的多个构象异构体
苔藓抑素 1(以下简称苔藓抑素)正处于治疗阿尔茨海默病和根除艾滋病毒/艾滋病的临床试验中。它也是癌症免疫疗法和其他治疗适应症的临床前先导。然而,对于膜微环境中苔藓抑素与其蛋白激酶 C (PKC) 靶标结合的构象一无所知。因此,设计更有效、耐受性更好或更容易合成的配体的努力仅限于不包括 PKC 或已知影响 PKC 配体结合的膜效应的结构。这个问题更普遍地延伸到人类蛋白质组中的许多膜相关蛋白。在这里,我们使用旋转回波双共振 (REDOR) 固态 NMR 来确定在磷脂囊泡存在的情况下与 PKCδ-C1b 结构域结合的 PKC 调节剂的构象。构象限制的 PKC 调节剂佛波醇二乙酸酯 (PDAc) 用作初始测试底物。虽然观察到 PDAc 在磷脂组件中的固定蛋白结合状态和移动状态之间发生意外分配,但鉴定出了结合状态的单一构象。与此形成鲜明对比的是,苔藓抑素类似物(bryolog)被发现仅以蛋白质结合状态存在,但采用由三个独立距离测量定义的构象分布。在功能相关的磷脂复合物中检测多个 PKCδ-C1b 结合的苔藓同构异构体揭示了细胞系统固有的动态性质,而单构象静态结构无法捕获这种性质。 这些结果表明,PKC 调节剂的结合、选择性和功能以及新调节剂的设计最好使用动态多态模型来解决,该分析可能适用于其他膜相关蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号