当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient interfacial charge transfer through plasmon sensitized Ag@Bi2O3 hierarchical photoanodes for photoelectrocatalytic degradation of chlorinated phenols
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7cp04888b Neerugatti KrishnaRao Eswar 1, 2, 3, 4 , Sangeeta Adhikari 2, 3, 4, 5 , Praveen C. Ramamurthy 1, 2, 3, 4 , Giridhar Madras 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7cp04888b Neerugatti KrishnaRao Eswar 1, 2, 3, 4 , Sangeeta Adhikari 2, 3, 4, 5 , Praveen C. Ramamurthy 1, 2, 3, 4 , Giridhar Madras 2, 3, 4, 5
Affiliation
|
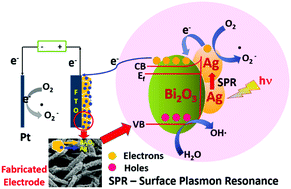
The present work demonstrates an extremely proficient and robust study of efficient interfacial charge transfer through plasmonic Ag decorated Bi2O3 hierarchical photoanodes for the photoelectrochemical treatment of chlorinated phenols. Unique 2D flake-like Bi2O3 hierarchical nanostructures were grown onto a fluorine-doped tin oxide (FTO) substrate by a simple chemical bath deposition method using triethanolamine as complexing agent. The formation of Bi2O3 on FTO was governed by the decomposition of a nucleated bismuth-hydroxyl complex (Bi2O1−x(OH)x) and modification to the electrode was carried out by the deposition of Ag via a chemical reduction method using hydrazine hydrate. Both the fabricated electrodes were well characterized for their photo- and electro-optical properties. Efficient charge separation was observed due to the surface plasmon resonance phenomenon of silver nanoparticles with the favorable intrinsic properties of Bi2O3 under application of a small electric bias of 1 V preventing the recombination of charge carriers and thereby increasing the rate of photoelectrocatalytic degradation of the chlorinated phenols. PEC degradation using the Ag@Bi2O3 photoelectrode followed the trend 4-CP < 2,4-DCP < 2,4,6-TCP < P-CP due to efficient attack at the chlorinated positions by reactive oxygen species with increasing chlorine substitution and also due to the absence of an expected chain reaction of the generated chlorine radicals (Cl˙) during the PEC reaction. The PEC activity of Ag@Bi2O3 was 1.5 times higher than a Bi2O3 nanoflake electrode for 4-CP over 2 h. The fabricated Ag@Bi2O3 proved to be an efficient photoelectrode with synergistic solar-induced photoactivity. A detailed mechanistic study in the presence of scavengers suggests degradation by produced hydroxyl radical species. Thus, physical insights into the degradation of chlorinated phenols were obtained.
中文翻译:

通过等离激元敏化的Ag @ Bi 2 O 3分级光阳极进行有效的界面电荷转移,以光催化降解氯化酚。
本工作证明了通过等离子体修饰的Ag装饰的Bi 2 O 3分级光阳极对氯酚进行光电化学处理的高效界面电荷转移的极其熟练和可靠的研究。通过使用三乙醇胺作为络合剂的简单化学浴沉积方法,将独特的2D片状Bi 2 O 3分层纳米结构生长在掺氟的氧化锡(FTO)衬底上。FTO上Bi 2 O 3的形成受有核铋-羟基配合物(Bi 2 O 1- x(OH)x)并通过使用水合肼的化学还原法沉积Ag来对电极进行修饰。两种制成的电极都具有良好的光和电光特性。观察到有效的电荷分离,这是由于在1 V的小电偏压下,具有Bi 2 O 3固有性质的银纳米粒子的表面等离子体共振现象,从而防止了电荷载流子的复合,从而提高了光催化降解ZnO的速率。氯化酚。使用Ag @ Bi 2 O 3降解PEC光电电极遵循4-CP <2,4-DCP <2,4,6-TCP <P-CP的趋势,这是由于活性氧在氯代位置对氯的有效攻击并增加了氯的取代,并且由于没有预期的在PEC反应过程中产生的氯自由基(Cl 3)的链式反应。在2小时内,Ag @ Bi 2 O 3的PEC活性比4-CP的Bi 2 O 3纳米薄片电极的PEC活性高1.5倍。人造Ag @ Bi 2 O 3被证明是一种具有协同作用的太阳光诱导活性的有效光电极。在清除剂存在下进行的详细机械研究表明,所产生的羟基自由基会降解。因此,获得了对氯化苯酚降解的物理见解。
更新日期:2018-01-02
中文翻译:

通过等离激元敏化的Ag @ Bi 2 O 3分级光阳极进行有效的界面电荷转移,以光催化降解氯化酚。
本工作证明了通过等离子体修饰的Ag装饰的Bi 2 O 3分级光阳极对氯酚进行光电化学处理的高效界面电荷转移的极其熟练和可靠的研究。通过使用三乙醇胺作为络合剂的简单化学浴沉积方法,将独特的2D片状Bi 2 O 3分层纳米结构生长在掺氟的氧化锡(FTO)衬底上。FTO上Bi 2 O 3的形成受有核铋-羟基配合物(Bi 2 O 1- x(OH)x)并通过使用水合肼的化学还原法沉积Ag来对电极进行修饰。两种制成的电极都具有良好的光和电光特性。观察到有效的电荷分离,这是由于在1 V的小电偏压下,具有Bi 2 O 3固有性质的银纳米粒子的表面等离子体共振现象,从而防止了电荷载流子的复合,从而提高了光催化降解ZnO的速率。氯化酚。使用Ag @ Bi 2 O 3降解PEC光电电极遵循4-CP <2,4-DCP <2,4,6-TCP <P-CP的趋势,这是由于活性氧在氯代位置对氯的有效攻击并增加了氯的取代,并且由于没有预期的在PEC反应过程中产生的氯自由基(Cl 3)的链式反应。在2小时内,Ag @ Bi 2 O 3的PEC活性比4-CP的Bi 2 O 3纳米薄片电极的PEC活性高1.5倍。人造Ag @ Bi 2 O 3被证明是一种具有协同作用的太阳光诱导活性的有效光电极。在清除剂存在下进行的详细机械研究表明,所产生的羟基自由基会降解。因此,获得了对氯化苯酚降解的物理见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号