Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Hydrolyzed Infant Formula vs Conventional Formula on Risk of Type 1 Diabetes
JAMA ( IF 63.1 ) Pub Date : 2018-01-02 , DOI: 10.1001/jama.2017.19826 , Mikael Knip 1, 2 , Hans K. Åkerblom 1 , Eva Al Taji 3 , Dorothy Becker 4 , Jan Bruining 5 , Luis Castano 6 , Thomas Danne 7 , Carine de Beaufort 8 , Hans-Michael Dosch 9 , John Dupre 10 , William D. Fraser 11 , Neville Howard 12 , Jorma Ilonen 13 , Daniel Konrad 14 , Olga Kordonouri 7 , Jeffrey P. Krischer 15 , Margaret L. Lawson 16 , Johnny Ludvigsson 17 , Laszlo Madacsy 18 , Jeffrey L. Mahon 10 , Anne Ormisson 19 , Jerry P. Palmer 20 , Paolo Pozzilli 21 , Erkki Savilahti 1 , Manuel Serrano-Rios 22 , Marco Songini 23 , Shayne Taback 24 , Outi Vaarala 1, 25 , Neil H. White 26 , Suvi M. Virtanen 27 , Renata Wasikowa 28
JAMA ( IF 63.1 ) Pub Date : 2018-01-02 , DOI: 10.1001/jama.2017.19826 , Mikael Knip 1, 2 , Hans K. Åkerblom 1 , Eva Al Taji 3 , Dorothy Becker 4 , Jan Bruining 5 , Luis Castano 6 , Thomas Danne 7 , Carine de Beaufort 8 , Hans-Michael Dosch 9 , John Dupre 10 , William D. Fraser 11 , Neville Howard 12 , Jorma Ilonen 13 , Daniel Konrad 14 , Olga Kordonouri 7 , Jeffrey P. Krischer 15 , Margaret L. Lawson 16 , Johnny Ludvigsson 17 , Laszlo Madacsy 18 , Jeffrey L. Mahon 10 , Anne Ormisson 19 , Jerry P. Palmer 20 , Paolo Pozzilli 21 , Erkki Savilahti 1 , Manuel Serrano-Rios 22 , Marco Songini 23 , Shayne Taback 24 , Outi Vaarala 1, 25 , Neil H. White 26 , Suvi M. Virtanen 27 , Renata Wasikowa 28
Affiliation
|
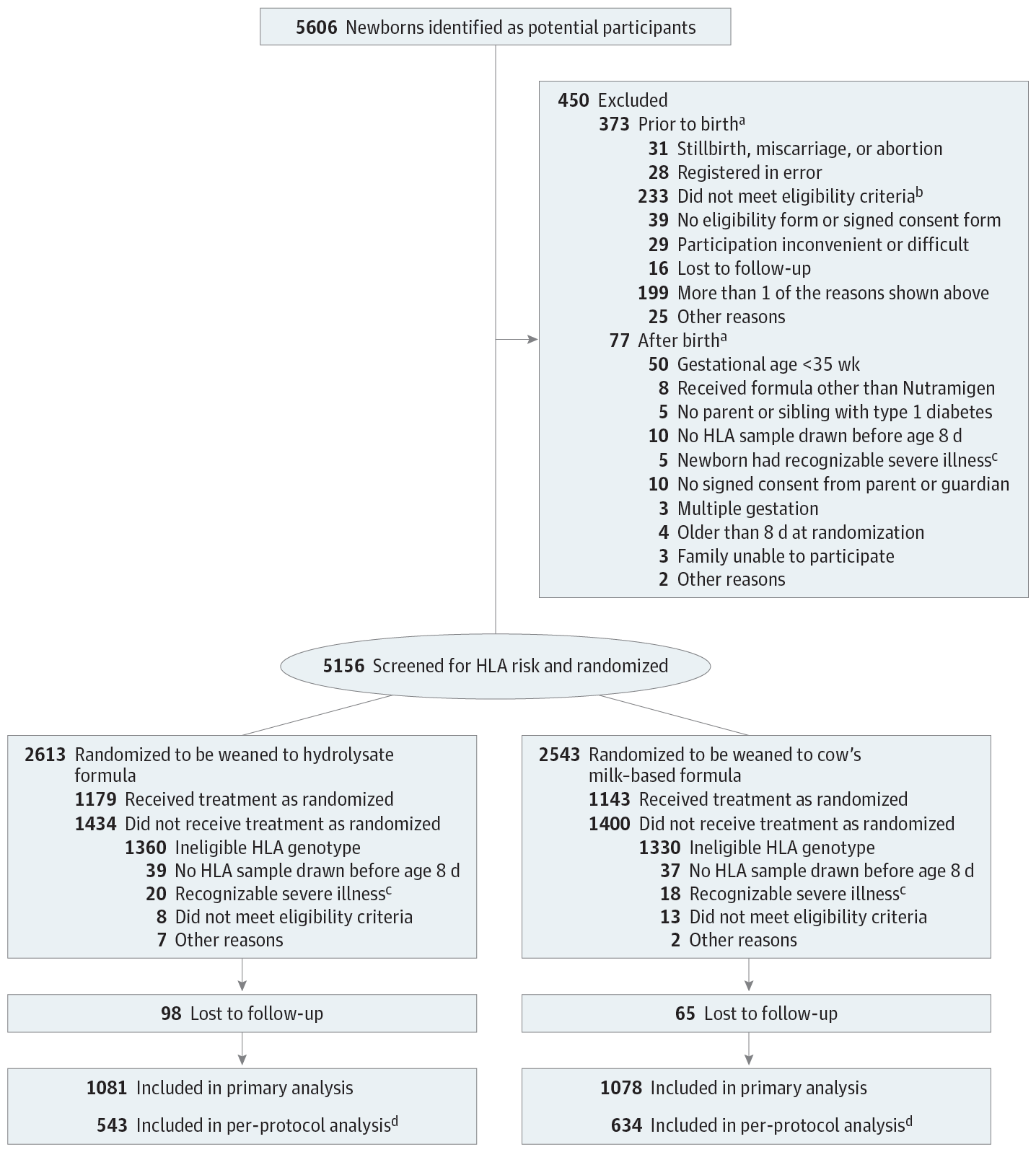
Importance Early exposure to complex dietary proteins may increase the risk of type 1 diabetes in children with genetic disease susceptibility. There are no intact proteins in extensively hydrolyzed formulas. Objective To test the hypothesis that weaning to an extensively hydrolyzed formula decreases the cumulative incidence of type 1 diabetes in young children. Design, Setting, and Participants An international double-blind randomized clinical trial of 2159 infants with human leukocyte antigen–conferred disease susceptibility and a first-degree relative with type 1 diabetes recruited from May 2002 to January 2007 in 78 study centers in 15 countries; 1081 were randomized to be weaned to the extensively hydrolyzed casein formula and 1078 to a conventional formula. The follow-up of the participants ended on February 28, 2017. Interventions The participants received either a casein hydrolysate or a conventional adapted cow’s milk formula supplemented with 20% of the casein hydrolysate. The minimum duration of study formula exposure was 60 days by 6 to 8 months of age. Main Outcomes and Measures Primary outcome was type 1 diabetes diagnosed according to World Health Organization criteria. Secondary outcomes included age at diabetes diagnosis and safety (adverse events). Results Among 2159 newborn infants (1021 female [47.3%]) who were randomized, 1744 (80.8%) completed the trial. The participants were observed for a median of 11.5 years (quartile [Q] 1-Q3, 10.2-12.8). The absolute risk of type 1 diabetes was 8.4% among those randomized to the casein hydrolysate (n = 91) vs 7.6% among those randomized to the conventional formula (n = 82) (difference, 0.8% [95% CI, −1.6% to 3.2%]). The hazard ratio for type 1 diabetes adjusted for human leukocyte antigen risk group, duration of breastfeeding, duration of study formula consumption, sex, and region while treating study center as a random effect was 1.1 (95% CI, 0.8 to 1.5; P = .46). The median age at diagnosis of type 1 diabetes was similar in the 2 groups (6.0 years [Q1-Q3, 3.1-8.9] vs 5.8 years [Q1-Q3, 2.6-9.1]; difference, 0.2 years [95% CI, −0.9 to 1.2]). Upper respiratory infections were the most common adverse event reported (frequency, 0.48 events/year in the hydrolysate group and 0.50 events/year in the control group). Conclusions and Relevance Among infants at risk for type 1 diabetes, weaning to a hydrolyzed formula compared with a conventional formula did not reduce the cumulative incidence of type 1 diabetes after median follow-up for 11.5 years. These findings do not support a need to revise the dietary recommendations for infants at risk for type 1 diabetes. Trial Registration clinicaltrials.gov Identifier: NCT00179777
中文翻译:

水解婴儿配方奶粉与传统配方奶粉对 1 型糖尿病风险的影响
重要性 早期接触复杂的膳食蛋白质可能会增加具有遗传病易感性的儿童患 1 型糖尿病的风险。深度水解配方中没有完整的蛋白质。目的 检验断奶至深度水解配方可降低幼儿 1 型糖尿病累积发病率的假设。设计、设置和参与者 2002 年 5 月至 2007 年 1 月在 15 个国家的 78 个研究中心招募了 2159 名患有人类白细胞抗原赋予疾病易感性的婴儿和 1 型糖尿病的一级亲属的国际双盲随机临床试验;1081 名被随机分配至完全水解酪蛋白配方奶粉,1078 名被随机分配至常规配方奶粉。参与者的随访于2017年2月28日结束。干预措施 参与者接受酪蛋白水解物或添加了 20% 酪蛋白水解物的常规改良牛奶配方。研究配方暴露的最短持续时间为 6 至 8 个月大时 60 天。主要结果和措施 主要结果是根据世界卫生组织标准诊断的 1 型糖尿病。次要结果包括糖尿病诊断时的年龄和安全性(不良事件)。结果 在随机分配的 2159 名新生儿(1021 名女性 [47.3%])中,1744 名(80.8%)完成了试验。参与者的观察中位数为 11.5 年(四分位数 [Q] 1-Q3,10.2-12.8)。随机分配至酪蛋白水解物的患者 (n = 91) 的 1 型糖尿病绝对风险为 8.4%,而随机分配至常规配方的患者 (n = 82) 的 1 型糖尿病绝对风险为 7.6%(差异,0.8% [95% CI,-1。6% 到 3.2%])。根据人类白细胞抗原风险组、母乳喂养持续时间、研究配方消费持续时间、性别和地区调整后的 1 型糖尿病风险比为 1.1(95% CI,0.8 至 1.5;P = .46)。两组诊断 1 型糖尿病时的中位年龄相似(6.0 岁 [Q1-Q3,3.1-8.9] vs 5.8 岁 [Q1-Q3,2.6-9.1];差异,0.2 岁 [95% CI,- 0.9 到 1.2])。上呼吸道感染是最常见的不良事件报告(频率,水解物组为 0.48 次事件/年,对照组为 0.50 次事件/年)。结论和相关性 在有 1 型糖尿病风险的婴儿中,在中位随访 11.5 年后,与传统配方奶粉相比,断奶至水解配方奶粉并未降低 1 型糖尿病的累积发病率。这些发现不支持修改对有 1 型糖尿病风险的婴儿的饮食建议的需要。试验注册clinicaltrials.gov 标识符:NCT00179777
更新日期:2018-01-02
中文翻译:

水解婴儿配方奶粉与传统配方奶粉对 1 型糖尿病风险的影响
重要性 早期接触复杂的膳食蛋白质可能会增加具有遗传病易感性的儿童患 1 型糖尿病的风险。深度水解配方中没有完整的蛋白质。目的 检验断奶至深度水解配方可降低幼儿 1 型糖尿病累积发病率的假设。设计、设置和参与者 2002 年 5 月至 2007 年 1 月在 15 个国家的 78 个研究中心招募了 2159 名患有人类白细胞抗原赋予疾病易感性的婴儿和 1 型糖尿病的一级亲属的国际双盲随机临床试验;1081 名被随机分配至完全水解酪蛋白配方奶粉,1078 名被随机分配至常规配方奶粉。参与者的随访于2017年2月28日结束。干预措施 参与者接受酪蛋白水解物或添加了 20% 酪蛋白水解物的常规改良牛奶配方。研究配方暴露的最短持续时间为 6 至 8 个月大时 60 天。主要结果和措施 主要结果是根据世界卫生组织标准诊断的 1 型糖尿病。次要结果包括糖尿病诊断时的年龄和安全性(不良事件)。结果 在随机分配的 2159 名新生儿(1021 名女性 [47.3%])中,1744 名(80.8%)完成了试验。参与者的观察中位数为 11.5 年(四分位数 [Q] 1-Q3,10.2-12.8)。随机分配至酪蛋白水解物的患者 (n = 91) 的 1 型糖尿病绝对风险为 8.4%,而随机分配至常规配方的患者 (n = 82) 的 1 型糖尿病绝对风险为 7.6%(差异,0.8% [95% CI,-1。6% 到 3.2%])。根据人类白细胞抗原风险组、母乳喂养持续时间、研究配方消费持续时间、性别和地区调整后的 1 型糖尿病风险比为 1.1(95% CI,0.8 至 1.5;P = .46)。两组诊断 1 型糖尿病时的中位年龄相似(6.0 岁 [Q1-Q3,3.1-8.9] vs 5.8 岁 [Q1-Q3,2.6-9.1];差异,0.2 岁 [95% CI,- 0.9 到 1.2])。上呼吸道感染是最常见的不良事件报告(频率,水解物组为 0.48 次事件/年,对照组为 0.50 次事件/年)。结论和相关性 在有 1 型糖尿病风险的婴儿中,在中位随访 11.5 年后,与传统配方奶粉相比,断奶至水解配方奶粉并未降低 1 型糖尿病的累积发病率。这些发现不支持修改对有 1 型糖尿病风险的婴儿的饮食建议的需要。试验注册clinicaltrials.gov 标识符:NCT00179777









































 京公网安备 11010802027423号
京公网安备 11010802027423号