当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AmPhos Pd-Catalyzed Suzuki–Miyaura Catalyst-Transfer Condensation Polymerization: Narrower Dispersity by Mixing the Catalyst and Base Prior to Polymerization
Macromolecules ( IF 5.1 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.macromol.7b01990 Kentaro Kosaka 1 , Tatsuya Uchida 1 , Koichiro Mikami 1 , Yoshihiro Ohta 1 , Tsutomu Yokozawa 1
Macromolecules ( IF 5.1 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.macromol.7b01990 Kentaro Kosaka 1 , Tatsuya Uchida 1 , Koichiro Mikami 1 , Yoshihiro Ohta 1 , Tsutomu Yokozawa 1
Affiliation
|
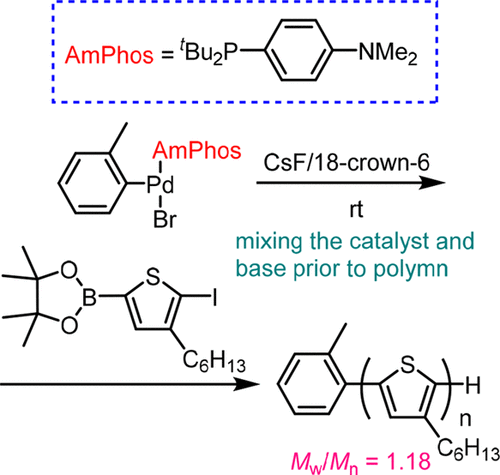
Several palladium catalysts with bulky phosphine ligands other than t-Bu3P were investigated for Suzuki–Miyaura catalyst-transfer condensation polymerization (CTCP). When the model reaction of 2,5-dibromothiophene with phenylboronic acid ester was carried out with a variety of Pd precatalysts in the presence of K3PO4 as a base, we found that the use of di-tert-butyl(4-dimethylaminophenyl)phosphine (AmPhos) Pd as a precatalyst resulted in exclusive formation of diphenyl-substituted thiophene. Polymerization of thiophene monomer and fluorene monomer with an initiator generated in situ from AmPhos Pd precatalyst and 4-iodobenzonitrile proceeded via the CTCP mechanism, affording polythiophene (P3HT) and polyfluorene with low dispersity and controlled polymer ends. Furthermore, (tolyl)PdAmPhos(Br) (7) was synthesized, and its polymerization properties were investigated. We found that polymerization of thiophene monomer with a mixture of 7 and CsF that had been stirred for 1 h prior to polymerization afforded P3HT with the dispersity of 1.18, which was considerably narrower than that in the case of CTCP with PhPd(t-Bu3P)Br. Block copolymerization of thiophene monomer and fluorene monomer with 7 proceeded irrespective of the polymerization order, in contrast to PhPd(t-Bu3P)Br-catalyzed block copolymerization, in which only chain extension with thiophene monomer from a polyfluorene propagating end took place and the reverse chain extension did not.
中文翻译:

AmPhos Pd催化的Suzuki-Miyaura催化剂转移缩合聚合:在聚合之前将催化剂和碱混合可得到更窄的分散度
铃木-Miyaura催化剂转移缩聚(CTCP)研究了几种叔丁基膦配体不同于t -Bu 3 P的钯催化剂。当2,5-二溴噻吩与苯基硼酸酯的模型反应用在K的存在的各种钯预催化剂的情况下进行3 PO 4作为碱,我们发现,使用二-叔-丁基(4-二甲基氨基苯基)膦(AmPhos)Pd作为前催化剂导致二苯基取代的噻吩的排他性形成。噻吩单体和芴单体与由AmPhos Pd预催化剂和4-碘代苄腈就地生成的引发剂的聚合通过CTCP机理进行,提供了具有低分散度和受控聚合物末端的聚噻吩(P3HT)和聚芴。此外,合成了(甲苯基)PdAmPhos(Br)(7),并研究了其聚合性能。我们发现噻吩单体与的混合物在聚合7已经用1.18分散度,这比在CTCP与PhPd的情况下窄得多聚合得到P3HT之前搅拌1个小时和CsF(吨-Bu3 P)溴。噻吩单体和芴单体与嵌段共聚7进行而不管聚合顺序的,而相比之下,PhPd(吨-Bu 3 P)BR-催化嵌段共聚,其中仅扩链噻吩单体从聚芴传播端发生和反向链延伸没有。
更新日期:2018-01-02
中文翻译:

AmPhos Pd催化的Suzuki-Miyaura催化剂转移缩合聚合:在聚合之前将催化剂和碱混合可得到更窄的分散度
铃木-Miyaura催化剂转移缩聚(CTCP)研究了几种叔丁基膦配体不同于t -Bu 3 P的钯催化剂。当2,5-二溴噻吩与苯基硼酸酯的模型反应用在K的存在的各种钯预催化剂的情况下进行3 PO 4作为碱,我们发现,使用二-叔-丁基(4-二甲基氨基苯基)膦(AmPhos)Pd作为前催化剂导致二苯基取代的噻吩的排他性形成。噻吩单体和芴单体与由AmPhos Pd预催化剂和4-碘代苄腈就地生成的引发剂的聚合通过CTCP机理进行,提供了具有低分散度和受控聚合物末端的聚噻吩(P3HT)和聚芴。此外,合成了(甲苯基)PdAmPhos(Br)(7),并研究了其聚合性能。我们发现噻吩单体与的混合物在聚合7已经用1.18分散度,这比在CTCP与PhPd的情况下窄得多聚合得到P3HT之前搅拌1个小时和CsF(吨-Bu3 P)溴。噻吩单体和芴单体与嵌段共聚7进行而不管聚合顺序的,而相比之下,PhPd(吨-Bu 3 P)BR-催化嵌段共聚,其中仅扩链噻吩单体从聚芴传播端发生和反向链延伸没有。











































 京公网安备 11010802027423号
京公网安备 11010802027423号