Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Synthesis of Morphan Derivatives by Michael and Hetero-Michael Addition of 1,1-Enediamines to Quinone Monoketals
ACS Omega ( IF 3.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acsomega.7b01493 Xing-Mei Hu 1 , Da-Yun Luo 1 , Quan-Xing Zi 1 , Jun Lin 1 , Sheng-Jiao Yan 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acsomega.7b01493 Xing-Mei Hu 1 , Da-Yun Luo 1 , Quan-Xing Zi 1 , Jun Lin 1 , Sheng-Jiao Yan 1
Affiliation
|
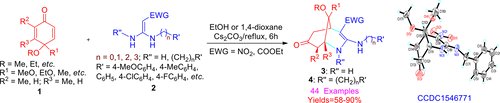
A general and concise method was developed for the synthesis of highly functionalized morphans 3–4 by the Michael and hetero-Michael addition reaction of different types of quinone monoketals 1 and 1,1-enediamines 2 in ethanol or 1,4-dioxane at reflux. This method is suitable for the efficient parallel syntheses of N-containing heterocycles. A library of highly functional morphan derivatives was easily constructed using the Michael/hetero-Michael reaction.
中文翻译:

迈克尔和1,1-烯二胺对醌单缩酮的非对映体合成吗啡基衍生物的非对映选择性
通过回流反应在乙醇或1,4-二恶烷中不同类型的醌单缩酮1和1,1-二烯胺2的迈克尔和杂合迈克尔加成反应,开发了一种通用且简洁的方法来合成高度官能化的吗啡3-4。该方法适合于含氮杂环的有效平行合成。使用Michael / hetero-Michael反应可轻松构建功能强大的吗啡衍生物库。
更新日期:2018-01-02
中文翻译:

迈克尔和1,1-烯二胺对醌单缩酮的非对映体合成吗啡基衍生物的非对映选择性
通过回流反应在乙醇或1,4-二恶烷中不同类型的醌单缩酮1和1,1-二烯胺2的迈克尔和杂合迈克尔加成反应,开发了一种通用且简洁的方法来合成高度官能化的吗啡3-4。该方法适合于含氮杂环的有效平行合成。使用Michael / hetero-Michael反应可轻松构建功能强大的吗啡衍生物库。











































 京公网安备 11010802027423号
京公网安备 11010802027423号