Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Circular Dichroisms of Mono- and Dibromo[2.2]paracyclophanes: A Combined Experimental and Theoretical Study
ACS Omega ( IF 4.1 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acsomega.7b01642 Mitsunobu Toda 1 , Yoshihisa Inoue 1 , Tadashi Mori 1
ACS Omega ( IF 4.1 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acsomega.7b01642 Mitsunobu Toda 1 , Yoshihisa Inoue 1 , Tadashi Mori 1
Affiliation
|
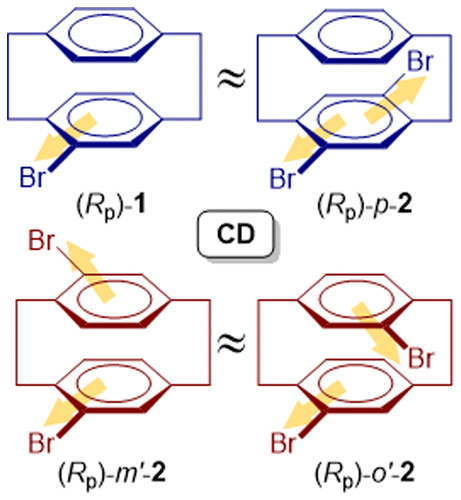
Circular dichroisms (CDs) of planar chiral 4-bromo[2.2]paracyclophane (1) and three isomeric dibromo[2.2]paracyclophanes (p-2, m′-2, and o′-2) were investigated experimentally and theoretically. They all exhibited strong multisignate Cotton effects (CEs) at the 1Lb, 1La, and 1B transitions of the component (bromo)benzene chromophore and were comparable to each other. For all of the cyclophanes examined, the enantiomer that eluted earlier from a chiral high-performance liquid chromatography column (Chiralcel IA or IB) exhibited negative and positive CEs at the 1Lb and 1La bands, respectively, which were followed by a more complicated pattern of CDs at the higher-energy bands. These CD features were well reproduced by quantum chemical calculations, allowing us to unambiguously assign the absolute configurations of the first-eluted enantiomers as Rp in all of the cases examined. Interestingly, the CDs of 1 and 2, although largely comparable in shape, were still sensitive to the number and pattern of bromine substitution, showing closer resemblance between m′-2 and o′-2 and between p-2 and 1. The theoretical calculations also reproduced successfully these spectral resemblance between them. The anisotropy (g) factors for the 1Lb bands of these cyclophanes were considerably large (∼10–2), whereas those for the 1La band were conventional in the order of 10–3. In addition, a weak CE was observed in the low-energy region at around 320 nm, which turned out to originate from the interplanar interaction and is hence assigned to the “cyclophane band”. The experimental g factors of this band were fairly large in the order of 10–2, but the computation turned out to be quite challenging and were less well reproduced theoretically, ascribable to the forbidden nature of the transition.
中文翻译:

单和二溴[2.2]对环粉的圆二色性:组合的实验和理论研究
平面手性4-溴[2.2]对环芳烷(的圆形dichroisms(CDS)1)和三个异构体二溴[2.2] paracyclophanes(p - 2,米' - 2,和ö ' - 2)实验和理论上进行了研究。它们在1 L b,1 L a和1 L处均表现出强大的多信号棉花效应(CEs)。组分(溴)苯生色团的B跃迁和彼此可比。对于所有检测的环烷,先前从手性高效液相色谱柱(Chiralcel IA或IB)洗脱的对映体分别在1 L b和1 L a谱带处显示阴性和阳性CE,随后是a高能带上CD的模式更加复杂。这些CD的特征通过量子化学计算得到了很好的再现,从而使我们能够在所有考察的情况下明确地将首次洗脱的对映异构体的绝对构型指定为R p。有趣的是,1和2的CD虽然在形状上基本上可比,仍然与数和溴取代的图案,示出之间更密切的相似性敏感米' - 2和Ö ' - 2之间p - 2和1。理论计算也成功地再现了它们之间的这些光谱相似性。各向异性(克)因子为1大号b这些环芳的条带是相当大的(〜10 -2),而那些用于1大号一个波段是在10顺序常规-3。另外,在320nm附近的低能区域中观察到弱的CE,事实证明其源自平面间的相互作用,因此被归为“环带”。该谱带的实验g因子相当大,大约为10 –2,但是计算结果极具挑战性,并且由于过渡的禁忌性质,理论上没有很好地重现。
更新日期:2018-01-02
中文翻译:

单和二溴[2.2]对环粉的圆二色性:组合的实验和理论研究
平面手性4-溴[2.2]对环芳烷(的圆形dichroisms(CDS)1)和三个异构体二溴[2.2] paracyclophanes(p - 2,米' - 2,和ö ' - 2)实验和理论上进行了研究。它们在1 L b,1 L a和1 L处均表现出强大的多信号棉花效应(CEs)。组分(溴)苯生色团的B跃迁和彼此可比。对于所有检测的环烷,先前从手性高效液相色谱柱(Chiralcel IA或IB)洗脱的对映体分别在1 L b和1 L a谱带处显示阴性和阳性CE,随后是a高能带上CD的模式更加复杂。这些CD的特征通过量子化学计算得到了很好的再现,从而使我们能够在所有考察的情况下明确地将首次洗脱的对映异构体的绝对构型指定为R p。有趣的是,1和2的CD虽然在形状上基本上可比,仍然与数和溴取代的图案,示出之间更密切的相似性敏感米' - 2和Ö ' - 2之间p - 2和1。理论计算也成功地再现了它们之间的这些光谱相似性。各向异性(克)因子为1大号b这些环芳的条带是相当大的(〜10 -2),而那些用于1大号一个波段是在10顺序常规-3。另外,在320nm附近的低能区域中观察到弱的CE,事实证明其源自平面间的相互作用,因此被归为“环带”。该谱带的实验g因子相当大,大约为10 –2,但是计算结果极具挑战性,并且由于过渡的禁忌性质,理论上没有很好地重现。



























 京公网安备 11010802027423号
京公网安备 11010802027423号