Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH and Surface Charge Switchability on Bifunctional Charge Gradients
Langmuir ( IF 3.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.langmuir.7b02334 Kayesh M. Ashraf 1 , Md Rezaul K. Khan 1 , Daniel A. Higgins 2 , Maryanne M. Collinson 1
Langmuir ( IF 3.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.langmuir.7b02334 Kayesh M. Ashraf 1 , Md Rezaul K. Khan 1 , Daniel A. Higgins 2 , Maryanne M. Collinson 1
Affiliation
|
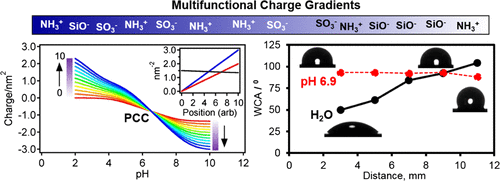
Multifunctionalized pH-sensitive silica gradients containing acidic and basic functional groups have been prepared to evaluate how the spatial arrangement of active sites on a surface influences the surface charge and pH switchability. The gradient surfaces were prepared using controlled rate infusion in such a manner that the individual gradients in the strong acid (sulfonic acid) and in the weak base (propylamine) align, whereas a gradient in the weakly acidic silanol groups opposes them. The relative amounts of the three species were varied by controlling the composition of the deposition solution, whereas the hydrophobicity of the underlying surface was set by using base layer-coated substrates prepared from either tetramethoxysilane or tetramethoxysilane/octyltrimethoxysilane mixtures. Results from X-ray photoelectron spectroscopy confirm that aligned gradients are formed in both amine and sulfonic acid groups, and the relative amounts bound to the surface follow that expected from the solution composition. Water contact angle measurements show a 40°–50° change across the length of the gradient, the exact values being dependent on the hydrophobicity of the base layer. Zeta potential measurements on gradient mimics reveal that there is a pH where the net charge on the gradient surface is predicted to have a constant but nonzero value. Static contact angle measurements and modeling confirm this prediction. At a pH acidic of this value, the gradient in charge runs in one direction, whereas at a pH basic of this value, the gradient in charge runs in the other direction. This point can be strategically moved from acidic values to basic values by changing the relative amounts of acidic and basic functionalities on the surface. The origin of this unique pH switchability can be found in acid–base chemistry. By modeling the charge along the gradient surface using a simple equilibrium model, a distribution of pKa values were noted in these materials.
中文翻译:

双功能电荷梯度的pH和表面电荷转换能力
已经制备了包含酸性和碱性官能团的多官能度pH敏感二氧化硅梯度,以评估表面活性位点的空间排列如何影响表面电荷和pH转换能力。使用控制速率的注入方式制备梯度表面,以使强酸(磺酸)和弱碱(丙胺)中的各个梯度对齐,而弱酸性硅烷醇基团中的梯度与之相反。通过控制沉积溶液的组成可以改变这三种物质的相对含量,而下表面的疏水性则是通过使用由四甲氧基硅烷或四甲氧基硅烷/辛基三甲氧基硅烷混合物制得的涂有基础层的基材来设定的。X射线光电子能谱的结果证实,在胺和磺酸基团中均形成了排列的梯度,并且结合至表面的相对量遵循溶液组成所预期的量。水接触角的测量结果表明,在整个梯度长度范围内,发生了40°–50°的变化,确切的值取决于基础层的疏水性。在梯度模拟物上的Zeta电势测量表明,存在一个pH值,其中梯度表面的净电荷被预测为具有恒定值,但非零。静态接触角测量和建模证实了这一预测。在此值的pH酸性下,电荷的梯度沿一个方向运行,而在此值的pH碱性下,电荷的梯度在另一方向上运行。通过改变表面上酸性和碱性官能团的相对含量,可以将该点从酸性值战略性地转移到碱性值。这种独特的pH转换能力的起源可以在酸碱化学中找到。通过使用简单的平衡模型对沿梯度表面的电荷进行建模,p的分布在这些材料中记录了K a值。
更新日期:2018-01-02
中文翻译:

双功能电荷梯度的pH和表面电荷转换能力
已经制备了包含酸性和碱性官能团的多官能度pH敏感二氧化硅梯度,以评估表面活性位点的空间排列如何影响表面电荷和pH转换能力。使用控制速率的注入方式制备梯度表面,以使强酸(磺酸)和弱碱(丙胺)中的各个梯度对齐,而弱酸性硅烷醇基团中的梯度与之相反。通过控制沉积溶液的组成可以改变这三种物质的相对含量,而下表面的疏水性则是通过使用由四甲氧基硅烷或四甲氧基硅烷/辛基三甲氧基硅烷混合物制得的涂有基础层的基材来设定的。X射线光电子能谱的结果证实,在胺和磺酸基团中均形成了排列的梯度,并且结合至表面的相对量遵循溶液组成所预期的量。水接触角的测量结果表明,在整个梯度长度范围内,发生了40°–50°的变化,确切的值取决于基础层的疏水性。在梯度模拟物上的Zeta电势测量表明,存在一个pH值,其中梯度表面的净电荷被预测为具有恒定值,但非零。静态接触角测量和建模证实了这一预测。在此值的pH酸性下,电荷的梯度沿一个方向运行,而在此值的pH碱性下,电荷的梯度在另一方向上运行。通过改变表面上酸性和碱性官能团的相对含量,可以将该点从酸性值战略性地转移到碱性值。这种独特的pH转换能力的起源可以在酸碱化学中找到。通过使用简单的平衡模型对沿梯度表面的电荷进行建模,p的分布在这些材料中记录了K a值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号