当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of a Potent, Highly Selective, and Brain Penetrant Phosphodiesterase 2A Inhibitor Clinical Candidate
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01466 Christopher J. Helal 1 , Eric Arnold 1 , Tracey Boyden 1 , Cheng Chang 1 , Thomas A. Chappie 2 , Ethan Fisher 1 , Mihaly Hajos 1 , John F. Harms 2 , William E. Hoffman 1 , John M. Humphrey 1 , Jayvardhan Pandit 1 , Zhijun Kang 1 , Robin J. Kleiman 1 , Bethany L. Kormos 2 , Che-Wah Lee 1 , Jiemin Lu 1 , Noha Maklad 1 , Laura McDowell 1 , Dina McGinnis 1 , Rebecca E. O’Connor 1 , Christopher J. O’Donnell 1 , Adam Ogden 1 , Mary Piotrowski 1 , Christopher J. Schmidt 2 , Patricia A. Seymour 1 , Hirokazu Ueno 1 , Nichole Vansell 1 , Patrick R. Verhoest 2 , Edward X. Yang 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01466 Christopher J. Helal 1 , Eric Arnold 1 , Tracey Boyden 1 , Cheng Chang 1 , Thomas A. Chappie 2 , Ethan Fisher 1 , Mihaly Hajos 1 , John F. Harms 2 , William E. Hoffman 1 , John M. Humphrey 1 , Jayvardhan Pandit 1 , Zhijun Kang 1 , Robin J. Kleiman 1 , Bethany L. Kormos 2 , Che-Wah Lee 1 , Jiemin Lu 1 , Noha Maklad 1 , Laura McDowell 1 , Dina McGinnis 1 , Rebecca E. O’Connor 1 , Christopher J. O’Donnell 1 , Adam Ogden 1 , Mary Piotrowski 1 , Christopher J. Schmidt 2 , Patricia A. Seymour 1 , Hirokazu Ueno 1 , Nichole Vansell 1 , Patrick R. Verhoest 2 , Edward X. Yang 1
Affiliation
|
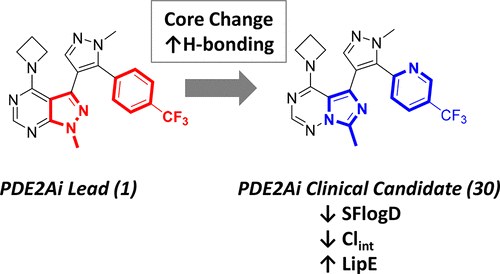
Computational modeling was used to direct the synthesis of analogs of previously reported phosphodiesterase 2A (PDE2A) inhibitor 1 with an imidazotriazine core to yield compounds of significantly enhanced potency. The analog PF-05180999 (30) was subsequently identified as a preclinical candidate targeting cognitive impairment associated with schizophrenia. Compound 30 demonstrated potent binding to PDE2A in brain tissue, dose responsive mouse brain cGMP increases, and reversal of N-methyl-d-aspartate (NMDA) antagonist-induced (MK-801, ketamine) effects in electrophysiology and working memory models in rats. Preclinical pharmacokinetics revealed unbound brain/unbound plasma levels approaching unity and good oral bioavailability resulting in an average concentration at steady state (Cav,ss) predicted human dose of 30 mg once daily (q.d.). Modeling of a modified release formulation suggested that 25 mg twice daily (b.i.d.) could maintain plasma levels of 30 at or above targeted efficacious plasma levels for 24 h, which became part of the human clinical plan.
中文翻译:

鉴定有力,高选择性和脑渗透性磷酸二酯酶2A抑制剂的临床候选药物
计算模型用于指导以前报道的磷酸二酯酶2A(PDE2A)抑制剂1与咪唑三嗪核心的类似物的合成,以产生效力显着增强的化合物。随后将类似物PF-05180999(30)确定为靶向与精神分裂症相关的认知障碍的临床前候选药物。化合物30在脑组织中显示出与PDE2A的强力结合,剂量反应性小鼠脑cGMP增加以及N-甲基-d的逆转天门冬氨酸(NMDA)拮抗剂诱导的(MK-801,氯胺酮)作用在大鼠的电生理和工作记忆模型中。临床前药物代谢动力学显示未结合的脑/未结合的血浆水平接近统一和良好的口服生物利用度,从而导致在稳定状态(平均浓度Ç AV,SS),每天一次(QD)预测的30毫克人剂量。调释制剂的模型表明,每天两次25 mg(出价)可以维持24小时内达到或高于目标有效血浆水平30的血浆水平,这已成为人类临床计划的一部分。
更新日期:2018-01-16
中文翻译:

鉴定有力,高选择性和脑渗透性磷酸二酯酶2A抑制剂的临床候选药物
计算模型用于指导以前报道的磷酸二酯酶2A(PDE2A)抑制剂1与咪唑三嗪核心的类似物的合成,以产生效力显着增强的化合物。随后将类似物PF-05180999(30)确定为靶向与精神分裂症相关的认知障碍的临床前候选药物。化合物30在脑组织中显示出与PDE2A的强力结合,剂量反应性小鼠脑cGMP增加以及N-甲基-d的逆转天门冬氨酸(NMDA)拮抗剂诱导的(MK-801,氯胺酮)作用在大鼠的电生理和工作记忆模型中。临床前药物代谢动力学显示未结合的脑/未结合的血浆水平接近统一和良好的口服生物利用度,从而导致在稳定状态(平均浓度Ç AV,SS),每天一次(QD)预测的30毫克人剂量。调释制剂的模型表明,每天两次25 mg(出价)可以维持24小时内达到或高于目标有效血浆水平30的血浆水平,这已成为人类临床计划的一部分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号