当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charging and Transport Dynamics of a Flow-Through Electrode Capacitive Deionization System
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.jpcb.7b09168 Yatian Qu 1, 2 , Patrick G. Campbell 2 , Ali Hemmatifar 1 , Jennifer M. Knipe 2 , Colin K. Loeb 2 , John J. Reidy 3 , Mckenzie A. Hubert 4 , Michael Stadermann 2 , Juan G. Santiago 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.jpcb.7b09168 Yatian Qu 1, 2 , Patrick G. Campbell 2 , Ali Hemmatifar 1 , Jennifer M. Knipe 2 , Colin K. Loeb 2 , John J. Reidy 3 , Mckenzie A. Hubert 4 , Michael Stadermann 2 , Juan G. Santiago 1
Affiliation
|
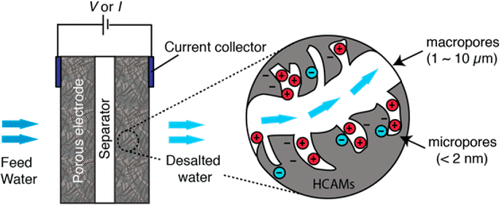
We present a study of the interplay among electric charging rate, capacitance, salt removal, and mass transport in “flow-through electrode” capacitive deionization (CDI) systems. We develop two models describing coupled transport and electro-adsorption/desorption which capture salt removal dynamics. The first model is a simplified, unsteady zero-dimensional volume-averaged model which identifies dimensionless parameters and figures of merits associated with cell performance. The second model is a higher fidelity area-averaged model which captures both spatial and temporal responses of charging. We further conducted an experimental study of these dynamics and considered two salt transport regimes: (1) advection-limited regime and (2) dispersion-limited regime. We use these data to validate models. The study shows that, in the advection-limited regime, differential charge efficiency determines the salt adsorption at the early stage of the deionization process. Subsequently, charging transitions to a quasi-steady state where salt removal rate is proportional to applied current scaled by the inlet flow rate. In the dispersion-dominated regime, differential charge efficiency, cell volume, and diffusion rates govern adsorption dynamics and flow rate has little effect. In both regimes, the interplay among mass transport rate, differential charge efficiency, cell capacitance, and (electric) charging current governs salt removal in flow-through electrode CDI.
中文翻译:

流通电极电容去离子系统的充电和传输动力学
我们对“流通电极”电容去离子(CDI)系统中的充电速率,电容,除盐和质量传输之间的相互作用进行了研究。我们开发了两个描述耦合运输和电吸附/解吸的模型,这些模型捕获了除盐动力学。第一个模型是简化的,不稳定的零维体积平均模型,该模型标识无量纲参数和与电池性能相关的优劣指标。第二个模型是更高保真度的面积平均模型,它捕获了充电的空间和时间响应。我们进一步对这些动力学进行了实验研究,并考虑了两种盐传输方式:(1)对流限制方式和(2)分散限制方式。我们使用这些数据来验证模型。研究表明,在对流限制的情况下,差电荷效率决定了去离子过程早期的盐吸附。随后,充电过渡到准稳态,其中除盐率与施加电流成比例,该电流由入口流量定标。在分散为主的体系中,差分电荷效率,电池体积和扩散速率决定了吸附动力学,流速几乎没有影响。在这两种情况下,质量传输速率,差分电荷效率,电池电容和(电)充电电流之间的相互作用决定着流通电极CDI中的盐去除。充注过渡到准稳态,其中除盐率与所施加的电流成比例,该电流由入口流量定标。在分散为主的体系中,差分电荷效率,电池体积和扩散速率决定了吸附动力学,流速几乎没有影响。在这两种情况下,质量传输速率,差分电荷效率,电池电容和(电)充电电流之间的相互作用决定着流通电极CDI中的盐去除。充注过渡到准稳态,其中除盐率与所施加的电流成比例,该电流由入口流量定标。在分散为主的体系中,差分电荷效率,电池体积和扩散速率决定了吸附动力学,流速几乎没有影响。在这两种情况下,质量传输速率,差分电荷效率,电池电容和(电)充电电流之间的相互作用决定着流通电极CDI中的盐去除。
更新日期:2018-01-02
中文翻译:

流通电极电容去离子系统的充电和传输动力学
我们对“流通电极”电容去离子(CDI)系统中的充电速率,电容,除盐和质量传输之间的相互作用进行了研究。我们开发了两个描述耦合运输和电吸附/解吸的模型,这些模型捕获了除盐动力学。第一个模型是简化的,不稳定的零维体积平均模型,该模型标识无量纲参数和与电池性能相关的优劣指标。第二个模型是更高保真度的面积平均模型,它捕获了充电的空间和时间响应。我们进一步对这些动力学进行了实验研究,并考虑了两种盐传输方式:(1)对流限制方式和(2)分散限制方式。我们使用这些数据来验证模型。研究表明,在对流限制的情况下,差电荷效率决定了去离子过程早期的盐吸附。随后,充电过渡到准稳态,其中除盐率与施加电流成比例,该电流由入口流量定标。在分散为主的体系中,差分电荷效率,电池体积和扩散速率决定了吸附动力学,流速几乎没有影响。在这两种情况下,质量传输速率,差分电荷效率,电池电容和(电)充电电流之间的相互作用决定着流通电极CDI中的盐去除。充注过渡到准稳态,其中除盐率与所施加的电流成比例,该电流由入口流量定标。在分散为主的体系中,差分电荷效率,电池体积和扩散速率决定了吸附动力学,流速几乎没有影响。在这两种情况下,质量传输速率,差分电荷效率,电池电容和(电)充电电流之间的相互作用决定着流通电极CDI中的盐去除。充注过渡到准稳态,其中除盐率与所施加的电流成比例,该电流由入口流量定标。在分散为主的体系中,差分电荷效率,电池体积和扩散速率决定了吸附动力学,流速几乎没有影响。在这两种情况下,质量传输速率,差分电荷效率,电池电容和(电)充电电流之间的相互作用决定着流通电极CDI中的盐去除。










































 京公网安备 11010802027423号
京公网安备 11010802027423号