当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decarboxylation-promoted Pd-catalyzed asymmetric propargylic [3 + 2] annulation for the enantioselective construction of a quaternary stereocenter in 2,3-dihydrofurans†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7ob02778h Kun Li 1, 2, 3, 4, 5 , Fu-Lin Zhu 4, 5, 6, 7 , Zhen-Ting Liu 4, 5, 6, 7 , Jing Tong 1, 2, 3, 4 , Xiang-Ping Hu 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7ob02778h Kun Li 1, 2, 3, 4, 5 , Fu-Lin Zhu 4, 5, 6, 7 , Zhen-Ting Liu 4, 5, 6, 7 , Jing Tong 1, 2, 3, 4 , Xiang-Ping Hu 4, 5, 6, 7
Affiliation
|
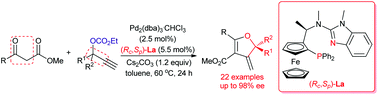
The enantioselective construction of a quaternary stereocenter in 2,3-dihydrofuran frameworks has been realized via the palladium-catalyzed asymmetric [3 + 2] cycloaddition of tertiary propargylic carbonates with β-ketoesters enabled by a chiral ferrocene/benzimidazole-based bidentate P,N-ligand. The reaction was significantly promoted by loss of CO2 to irreversibly form π-propargylpalladium or allenylpalladium intermediates. This protocol features a good tolerance of functional groups in both tertiary propargylic carbonates and β-ketoesters, thereby delivering a variety of highly functionalized chiral 2,3-dihydrofurans bearing a quaternary stereocenter at the 2-position and an exocyclic double bond at the 3-position in good chemical yields and high enantioselectivities (up to 98% ee).
中文翻译:

脱羧促进的Pd催化的不对称炔丙基[3 + 2]环化反应,用于2,3-二氢呋喃中四元立体中心的对映选择性构建†
季立构的2,3-二氢呋喃的框架对映结构已经实现通过钯催化的不对称[3 + 2]环加成叔炔碳酸酯与β酮酯能够通过手性二茂铁/苯并咪唑为基础的二齿P,N -配体。CO 2的损失显着促进了反应不可逆地形成π-炔丙基钯或烯丙基钯中间体。该方案的特点是对叔炔丙基碳酸酯和β-酮酸酯中的官能团具有良好的耐受性,从而提供了各种高度官能化的手性2,3-二氢呋喃,其2-位带有一个立体立体中心,而3-位带有一个环外双键。具有良好的化学收率和高对映选择性(高达98%ee)的位置。
更新日期:2018-01-02
中文翻译:

脱羧促进的Pd催化的不对称炔丙基[3 + 2]环化反应,用于2,3-二氢呋喃中四元立体中心的对映选择性构建†
季立构的2,3-二氢呋喃的框架对映结构已经实现通过钯催化的不对称[3 + 2]环加成叔炔碳酸酯与β酮酯能够通过手性二茂铁/苯并咪唑为基础的二齿P,N -配体。CO 2的损失显着促进了反应不可逆地形成π-炔丙基钯或烯丙基钯中间体。该方案的特点是对叔炔丙基碳酸酯和β-酮酸酯中的官能团具有良好的耐受性,从而提供了各种高度官能化的手性2,3-二氢呋喃,其2-位带有一个立体立体中心,而3-位带有一个环外双键。具有良好的化学收率和高对映选择性(高达98%ee)的位置。











































 京公网安备 11010802027423号
京公网安备 11010802027423号