当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A DFT study of the adsorption of short peptides on Mg and Mg-based alloy surfaces
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7cp07431j Zhe Fang 1, 2, 3, 4 , Jianfeng Wang 1, 2, 3, 4 , Shijie Zhu 1, 2, 3, 4 , Xiaofan Yang 4, 5, 6 , Yu Jia 4, 7, 8, 9, 10 , Qiang Sun 2, 3, 4, 10 , Shaokang Guan 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7cp07431j Zhe Fang 1, 2, 3, 4 , Jianfeng Wang 1, 2, 3, 4 , Shijie Zhu 1, 2, 3, 4 , Xiaofan Yang 4, 5, 6 , Yu Jia 4, 7, 8, 9, 10 , Qiang Sun 2, 3, 4, 10 , Shaokang Guan 1, 2, 3, 4
Affiliation
|
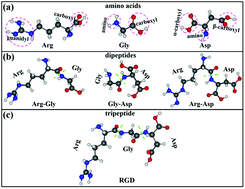
Adsorption of short peptides, including three dipeptides: arginine–glycine (Arg–Gly), glycine–aspartic acid (Gly–Asp), arginine–aspartic acid (Arg–Asp), and one tripeptide arginine–glycine–aspartic acid (RGD), on the surfaces of Mg and Mg alloys (Mg–Zn, Mg–Y, and Mg–Nd), was studied using the first-principles calculations based on density functional theory (DFT), considering van der Waals (vdW) correction. The calculated adsorption energies (Eads) of short peptides on the clean Mg(0001) surface are in the range of −1.73 to −2.80 eV per dipeptide, and −3.24 eV for RGD. The short peptides prefer to bond to Mg atoms at the surface by the O and N anions in their functional groups. For the clean Mg(0001) surface, the Eads of the short peptides are exclusively dominated by the number of functional groups binding to the surface. However, for the surface of the Mg–Zn alloy (1% Zn), the adsorption of the peptides is clearly enhanced (by about 0.3 eV per peptide) due to the enhanced N–Mg bond and the electrostatic interactions between the doped Zn at the surface and the backbone chains of the peptides. Furthermore, the attractive interactions are increased with the increase of doped Zn contents (up to 3%). In contrast, for the surfaces of Mg–Y (1% Y) and Mg–Nd (1% Nd) alloys, the adsorption of the peptides is slightly weakened compared to that on the clean Mg(0001) surfaces. Our results provide useful guidance in understanding the interactions between peptides and the Mg-based biomedical alloy surfaces at the atomic scale in the biomimetic coating fields.
中文翻译:

DFT研究短肽在Mg和Mg基合金表面上的吸附
短肽的吸附,包括三种二肽:精氨酸-甘氨酸(Arg-Gly),甘氨酸-天冬氨酸(Gly-Asp),精氨酸-天冬氨酸(Arg-Asp)和一种三肽精氨酸-甘氨酸-天冬氨酸(RGD)在Mg和Mg合金(Mg–Zn,Mg–Y和Mg–Nd)的表面上,使用基于密度泛函理论(DFT)的第一原理计算方法,并考虑了范德华(vdW)校正,研究了Mg和Mg合金的表面。所计算出的吸附能(Ë广告上的清洁镁短肽)(0001)面是在每二肽-1.73至-2.80电子伏特的范围内,和-3.24电子伏特为RGD。短肽倾向于通过其官能团中的O和N阴离子与表面的Mg原子键合。对于干净的Mg(0001)表面,E广告短肽中的大部分仅由结合至表面的官能团的数量决定。但是,对于镁锌合金的表面(锌含量为1%),由于增强的N-Mg键和掺杂的Zn之间的静电相互作用,肽的吸附明显增强(每个肽约0.3 eV)。肽的表面和主链。此外,随着掺杂锌含量(高达3%)的增加,吸引力相互作用也增加了。相比之下,对于Mg-Y(1%Y)和Mg-Nd(1%Nd)合金的表面,与干净的Mg(0001)表面相比,肽的吸附稍有减弱。我们的结果为了解肽和仿生涂层领域中原子级的基于Mg的生物医学合金表面之间的相互作用提供了有用的指导。
更新日期:2018-01-02
中文翻译:

DFT研究短肽在Mg和Mg基合金表面上的吸附
短肽的吸附,包括三种二肽:精氨酸-甘氨酸(Arg-Gly),甘氨酸-天冬氨酸(Gly-Asp),精氨酸-天冬氨酸(Arg-Asp)和一种三肽精氨酸-甘氨酸-天冬氨酸(RGD)在Mg和Mg合金(Mg–Zn,Mg–Y和Mg–Nd)的表面上,使用基于密度泛函理论(DFT)的第一原理计算方法,并考虑了范德华(vdW)校正,研究了Mg和Mg合金的表面。所计算出的吸附能(Ë广告上的清洁镁短肽)(0001)面是在每二肽-1.73至-2.80电子伏特的范围内,和-3.24电子伏特为RGD。短肽倾向于通过其官能团中的O和N阴离子与表面的Mg原子键合。对于干净的Mg(0001)表面,E广告短肽中的大部分仅由结合至表面的官能团的数量决定。但是,对于镁锌合金的表面(锌含量为1%),由于增强的N-Mg键和掺杂的Zn之间的静电相互作用,肽的吸附明显增强(每个肽约0.3 eV)。肽的表面和主链。此外,随着掺杂锌含量(高达3%)的增加,吸引力相互作用也增加了。相比之下,对于Mg-Y(1%Y)和Mg-Nd(1%Nd)合金的表面,与干净的Mg(0001)表面相比,肽的吸附稍有减弱。我们的结果为了解肽和仿生涂层领域中原子级的基于Mg的生物医学合金表面之间的相互作用提供了有用的指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号