当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interplay of Lewis acidity, intramolecular O→Sn interactions and selectivity: organotin-functionalized crown ethers as ditopic hosts for sodium and potassium halides
Chemical Communications ( IF 4.3 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7cc09263f Alain Charly Tagne Kuate 1, 2, 3, 4, 5 , Muhammad Moazzam Naseer 1, 2, 3, 4, 5 , Michael Lutter 1, 2, 3, 4 , Klaus Jurkschat 1, 2, 3, 4
Chemical Communications ( IF 4.3 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1039/c7cc09263f Alain Charly Tagne Kuate 1, 2, 3, 4, 5 , Muhammad Moazzam Naseer 1, 2, 3, 4, 5 , Michael Lutter 1, 2, 3, 4 , Klaus Jurkschat 1, 2, 3, 4
Affiliation
|
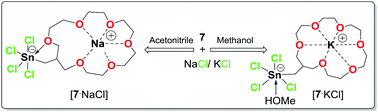
The increased Lewis acidity in organotin-functionalized crown ethers X3SnCH2[19]-crown-6 (5, X = I; 6, X = Br; 7, X = Cl) not only resulted in ditopic complexation of sodium/potassium halides, but also offers an excellent strategy to manipulate through intramolecular O→Sn interactions the selectivity of the crown ether moiety towards Na+/K+ depending on the solvent.
中文翻译:

Lewis酸度,分子内O→Sn相互作用和选择性之间的相互作用:有机锡官能化冠醚是卤化钠和卤化钾的二位主体
有机锡官能化冠醚X 3 SnCH 2 [19] -crown-6(5,X = I; 6,X = Br; 7,X = Cl)中路易斯酸的增加不仅导致钠/钾的二位络合卤化物,但也提供了一种出色的策略,可通过分子内O→Sn相互作用控制冠醚部分对Na + / K +的选择性,具体取决于溶剂。
更新日期:2018-01-18
中文翻译:

Lewis酸度,分子内O→Sn相互作用和选择性之间的相互作用:有机锡官能化冠醚是卤化钠和卤化钾的二位主体
有机锡官能化冠醚X 3 SnCH 2 [19] -crown-6(5,X = I; 6,X = Br; 7,X = Cl)中路易斯酸的增加不仅导致钠/钾的二位络合卤化物,但也提供了一种出色的策略,可通过分子内O→Sn相互作用控制冠醚部分对Na + / K +的选择性,具体取决于溶剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号