当前位置:
X-MOL 学术
›
Anal. Chim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Green-modified micellar liquid chromatography for isocratic isolation of some cardiovascular drugs with different polarities through experimental design approach
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.aca.2017.12.021 Amir M. Ramezani , Ghodratollah Absalan , Raheleh Ahmadi
Analytica Chimica Acta ( IF 5.7 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.aca.2017.12.021 Amir M. Ramezani , Ghodratollah Absalan , Raheleh Ahmadi
|
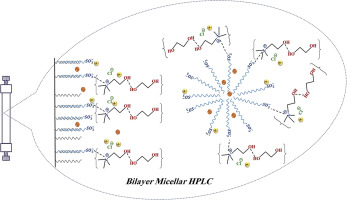
Bilayer pseudo-stationary phase micellar liquid chromatography (MLC) was developed for simultaneous isocratic isolation of hydrochlorothiazide, as a basic-polar (hydrophilic) cardiovascular drug, as well as triamterene and losartan potassium, as acidic-nonpolar (hydrophobic) cardiovascular drugs. Utilizing a deep eutectic solvent (DES), as a novel green mobile phase additive in combination with acetonitrile (ACN) and acetic acid (ACA), drastically improved the chromatographic behavior of the drugs. Concentration of sodium dodecyl sulphate (SDS), as well as volume percentages of ACN, DES, and ACA were optimized by using a central composite design. The optimal composition of the mobile phase (0.12 mol L-1 SDS, 5% ACN, 4% DES, and 2% ACA) was chosen through the desirability function. The chromatographic peaks of both hydrophilic and hydrophobic drugs, respectively, emerged at high and low retention time values in the shortest total analysis time of 20 min (at a flow rate of 2 mL min-1). Analytical characterization of the developed approach was investigated through Food and Drug Administration (FDA) guidelines. Applicability of the method was evaluated by analysing of human plasma samples which were directly injected into the system.
中文翻译:

绿色改性胶束液相色谱法通过实验设计方法等度分离一些不同极性的心血管药物
双层假固定相胶束液相色谱 (MLC) 被开发用于同时等度分离氢氯噻嗪,作为碱性极性(亲水性)心血管药物,以及氨苯蝶啶和氯沙坦钾,作为酸性-非极性(疏水性)心血管药物。利用深共熔溶剂 (DES) 作为一种新型绿色流动相添加剂,与乙腈 (ACN) 和乙酸 (ACA) 结合使用,显着改善了药物的色谱行为。使用中心复合设计优化了十二烷基硫酸钠 (SDS) 的浓度以及 ACN、DES 和 ACA 的体积百分比。通过合意性函数选择流动相的最佳组成(0.12 mol L-1 SDS、5% ACN、4% DES 和 2% ACA)。亲水性和疏水性药物的色谱峰,在 20 分钟的最短总分析时间内(流速为 2 mL min-1)分别出现在高和低保留时间值处。通过食品和药物管理局 (FDA) 指南对所开发方法的分析表征进行了调查。通过分析直接注入系统的人血浆样品来评估该方法的适用性。
更新日期:2018-06-01
中文翻译:

绿色改性胶束液相色谱法通过实验设计方法等度分离一些不同极性的心血管药物
双层假固定相胶束液相色谱 (MLC) 被开发用于同时等度分离氢氯噻嗪,作为碱性极性(亲水性)心血管药物,以及氨苯蝶啶和氯沙坦钾,作为酸性-非极性(疏水性)心血管药物。利用深共熔溶剂 (DES) 作为一种新型绿色流动相添加剂,与乙腈 (ACN) 和乙酸 (ACA) 结合使用,显着改善了药物的色谱行为。使用中心复合设计优化了十二烷基硫酸钠 (SDS) 的浓度以及 ACN、DES 和 ACA 的体积百分比。通过合意性函数选择流动相的最佳组成(0.12 mol L-1 SDS、5% ACN、4% DES 和 2% ACA)。亲水性和疏水性药物的色谱峰,在 20 分钟的最短总分析时间内(流速为 2 mL min-1)分别出现在高和低保留时间值处。通过食品和药物管理局 (FDA) 指南对所开发方法的分析表征进行了调查。通过分析直接注入系统的人血浆样品来评估该方法的适用性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号