当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the Role of the Hv1 C-Terminal Domain in Dimer Stabilization
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpcb.7b08669 Panisak Boonamnaj 1 , Pornthep Sompornpisut 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpcb.7b08669 Panisak Boonamnaj 1 , Pornthep Sompornpisut 1
Affiliation
|
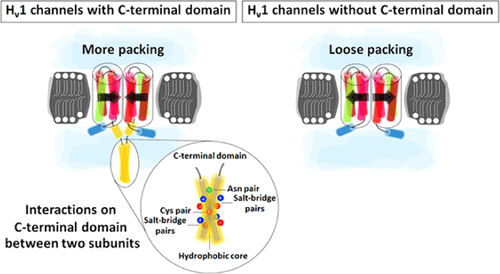
The voltage-gated proton-selective channel (Hv1) conducts protons in response to changes in membrane potential. The Hv1 protein forms dimers in the membrane. Crystal structures of Hv1 channels have revealed that the primary contacts between the two monomers are in the C-terminal domain (CTD), which forms a coiled-coil structure. The role of Hv1-CTD in channel assembly and activity is not fully understood. Here, molecular dynamics (MD) simulations of full-length and truncated CTD models of human and mouse Hv1 channels reveal a strong contribution of the CTD to the packing of the transmembrane domains. Simulations of the CTD models highlight four fundamental interactions of the key residues contributing to dimer stability. These include salt bridges, hydrophobic interactions, hydrogen bonds, and a disulfide bond across the dimer interface. At neutral pH, salt-bridge interactions increase dimer stability and the dimer becomes less stable at acidic pH. Hydrophobic core packing of the heptad pattern is important for stability, as shown by favorable nonpolar binding free energies rather than by electrostatic components. Moreover, free-energy calculations indicate that a more uniform hydrophobic core in the coiled-coil structure of the Hv1-NIN, a channel carrying the triple mutation M234N–N235I–V236N, leads to an increase in dimer stability with respect to the wild-type. A Cys disulfide bond has a strong impact on dimer stability by holding the dimer together and facilitating the interactions described above. These results are consistent with dissociative temperatures and energy barriers of dimer dissociation obtained from the temperature-accelerated MD.
中文翻译:

深入了解 Hv1 C 端域在二聚体稳定中的作用
电压门控质子选择性通道 (Hv1) 传导质子以响应膜电位的变化。Hv1 蛋白在膜中形成二聚体。Hv1 通道的晶体结构表明,两种单体之间的主要接触位于 C 端结构域 (CTD),形成卷曲螺旋结构。Hv1-CTD 在通道组装和活动中的作用尚不完全清楚。在这里,人类和小鼠 Hv1 通道的全长和截断 CTD 模型的分子动力学 (MD) 模拟揭示了 CTD 对跨膜域包装的重要贡献。CTD 模型的模拟突出了有助于二聚体稳定性的关键残基的四种基本相互作用。这些包括盐桥、疏水相互作用、氢键和跨二聚体界面的二硫键。在中性 pH 值下,盐桥相互作用增加了二聚体的稳定性,而二聚体在酸性 pH 下变得不太稳定。七元模式的疏水核心堆积对于稳定性很重要,如有利的非极性结合自由能而不是静电成分所示。此外,自由能计算表明,Hv1-NIN 的卷曲螺旋结构中更均匀的疏水核心,携带三重突变 M234N-N235I-V236N 的通道,导致二聚体稳定性增加。类型。Cys 二硫键通过将二聚体结合在一起并促进上述相互作用对二聚体稳定性有很大影响。这些结果与从温度加速的 MD 获得的二聚体解离的解离温度和能量势垒一致。盐桥相互作用增加了二聚体的稳定性,二聚体在酸性 pH 下变得不稳定。七元模式的疏水核心堆积对于稳定性很重要,如有利的非极性结合自由能而不是静电成分所示。此外,自由能计算表明,Hv1-NIN 的卷曲螺旋结构中更均匀的疏水核心,携带三重突变 M234N-N235I-V236N 的通道,导致二聚体稳定性增加。类型。Cys 二硫键通过将二聚体结合在一起并促进上述相互作用对二聚体稳定性有很大影响。这些结果与从温度加速的 MD 获得的二聚体解离的解离温度和能量势垒一致。盐桥相互作用增加了二聚体的稳定性,二聚体在酸性 pH 下变得不稳定。七元模式的疏水核心堆积对于稳定性很重要,如有利的非极性结合自由能而不是静电成分所示。此外,自由能计算表明,Hv1-NIN 的卷曲螺旋结构中更均匀的疏水核心,携带三重突变 M234N-N235I-V236N 的通道,导致二聚体稳定性增加。类型。Cys 二硫键通过将二聚体结合在一起并促进上述相互作用对二聚体稳定性有很大影响。这些结果与从温度加速的 MD 获得的二聚体解离的解离温度和能量势垒一致。
更新日期:2018-01-16
中文翻译:

深入了解 Hv1 C 端域在二聚体稳定中的作用
电压门控质子选择性通道 (Hv1) 传导质子以响应膜电位的变化。Hv1 蛋白在膜中形成二聚体。Hv1 通道的晶体结构表明,两种单体之间的主要接触位于 C 端结构域 (CTD),形成卷曲螺旋结构。Hv1-CTD 在通道组装和活动中的作用尚不完全清楚。在这里,人类和小鼠 Hv1 通道的全长和截断 CTD 模型的分子动力学 (MD) 模拟揭示了 CTD 对跨膜域包装的重要贡献。CTD 模型的模拟突出了有助于二聚体稳定性的关键残基的四种基本相互作用。这些包括盐桥、疏水相互作用、氢键和跨二聚体界面的二硫键。在中性 pH 值下,盐桥相互作用增加了二聚体的稳定性,而二聚体在酸性 pH 下变得不太稳定。七元模式的疏水核心堆积对于稳定性很重要,如有利的非极性结合自由能而不是静电成分所示。此外,自由能计算表明,Hv1-NIN 的卷曲螺旋结构中更均匀的疏水核心,携带三重突变 M234N-N235I-V236N 的通道,导致二聚体稳定性增加。类型。Cys 二硫键通过将二聚体结合在一起并促进上述相互作用对二聚体稳定性有很大影响。这些结果与从温度加速的 MD 获得的二聚体解离的解离温度和能量势垒一致。盐桥相互作用增加了二聚体的稳定性,二聚体在酸性 pH 下变得不稳定。七元模式的疏水核心堆积对于稳定性很重要,如有利的非极性结合自由能而不是静电成分所示。此外,自由能计算表明,Hv1-NIN 的卷曲螺旋结构中更均匀的疏水核心,携带三重突变 M234N-N235I-V236N 的通道,导致二聚体稳定性增加。类型。Cys 二硫键通过将二聚体结合在一起并促进上述相互作用对二聚体稳定性有很大影响。这些结果与从温度加速的 MD 获得的二聚体解离的解离温度和能量势垒一致。盐桥相互作用增加了二聚体的稳定性,二聚体在酸性 pH 下变得不稳定。七元模式的疏水核心堆积对于稳定性很重要,如有利的非极性结合自由能而不是静电成分所示。此外,自由能计算表明,Hv1-NIN 的卷曲螺旋结构中更均匀的疏水核心,携带三重突变 M234N-N235I-V236N 的通道,导致二聚体稳定性增加。类型。Cys 二硫键通过将二聚体结合在一起并促进上述相互作用对二聚体稳定性有很大影响。这些结果与从温度加速的 MD 获得的二聚体解离的解离温度和能量势垒一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号