当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Characterization and Electrocatalytic Application of Gold Nanoparticles Synthesized with Different Stabilizing Agents
Electroanalysis ( IF 2.7 ) Pub Date : 2017-12-29 , DOI: 10.1002/elan.201700633 Paulina Sierra-Rosales 1 , Rodrigo Torres 2 , Carlos Sepúlveda 2 , Marcelo J. Kogan 2, 3 , Juan Arturo Squella 4
Electroanalysis ( IF 2.7 ) Pub Date : 2017-12-29 , DOI: 10.1002/elan.201700633 Paulina Sierra-Rosales 1 , Rodrigo Torres 2 , Carlos Sepúlveda 2 , Marcelo J. Kogan 2, 3 , Juan Arturo Squella 4
Affiliation
|
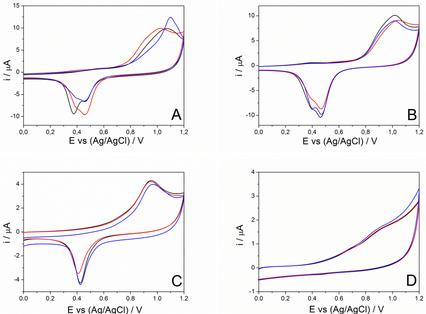
Gold nanoparticles (AuNPs) have unique properties, making them attractive for electronic and energyconversion devices and as (electro)catalysts for electrochemical sensors. In addition to the size and shape of AuNPs, the electrocatalytic properties of AuNP-sensors are also determined by the stabilizing agent used in their synthesis. Here, AuNPs were synthesized with citrate, alginate and quercetin, obtaining spherical and negatively charged nanoparticles. The AuNPs were used to modify glassy carbon electrodes (AuNPs/GCE), which were characterized by scanning electron microscopy and electrochemical techniques. The AuNPs/GCE showed aggregates of different sizes and degrees of dispersion on the electrode surface depending on the stabilizing agent. The AuNP’s aggregates affect the homogeneity of the film, the reproducibility of the electrodes and their response in buffer solution. Finally, to evaluate the electrocatalytic ability of the AuNPs/GCE, we studied the oxidation of two analytes with opposite charges: (1) sunset yellow (negative) and (2) hydrazine (positive). Compared with GCE, the AuNPs/GCE showed good electrocatalytic properties for hydrazine, increasing the current up to 50 % and shifting the potential by almost 400 mV, depending on the AuNP used. For the negatively charged analyte, the current decreased up to 50% and no shift in potential was observed. Thus, the electrocatalytic properties of the AuNPs showed to be highly dependent on the nature of the analyte.
中文翻译:

不同稳定剂合成金纳米粒子的电化学表征及电催化应用
金纳米粒子 (AuNPs) 具有独特的性质,使其对电子和能量转换设备以及作为电化学传感器的(电)催化剂具有吸引力。除了 AuNPs 的大小和形状之外,AuNP 传感器的电催化性能还取决于其合成中使用的稳定剂。在这里,AuNPs 与柠檬酸盐、藻酸盐和槲皮素合成,获得球形和带负电荷的纳米粒子。AuNPs用于修饰玻碳电极(AuNPs/GCE),通过扫描电子显微镜和电化学技术对其进行表征。根据稳定剂的不同,AuNPs/GCE 在电极表面显示出不同尺寸和分散程度的聚集体。AuNP 的聚集体影响薄膜的均匀性,电极的重现性及其在缓冲溶液中的响应。最后,为了评估 AuNPs/GCE 的电催化能力,我们研究了两种具有相反电荷的分析物的氧化:(1)日落黄(负)和(2)肼(正)。与 GCE 相比,AuNPs/GCE 对肼显示出良好的电催化性能,根据所使用的 AuNP,将电流增加至 50%,并将电位移动近 400 mV。对于带负电的分析物,电流降低了 50%,并且没有观察到电位变化。因此,AuNPs 的电催化性能高度依赖于分析物的性质。(1)日落黄(阴性)和(2)肼(阳性)。与 GCE 相比,AuNPs/GCE 对肼显示出良好的电催化性能,根据所使用的 AuNP,将电流增加至 50%,并将电位移动近 400 mV。对于带负电的分析物,电流降低了 50%,并且没有观察到电位变化。因此,AuNPs 的电催化性能高度依赖于分析物的性质。(1)日落黄(阴性)和(2)肼(阳性)。与 GCE 相比,AuNPs/GCE 对肼显示出良好的电催化性能,根据所使用的 AuNP,将电流增加至 50%,并将电位移动近 400 mV。对于带负电的分析物,电流降低了 50%,并且没有观察到电位变化。因此,AuNPs 的电催化性能高度依赖于分析物的性质。
更新日期:2017-12-29
中文翻译:

不同稳定剂合成金纳米粒子的电化学表征及电催化应用
金纳米粒子 (AuNPs) 具有独特的性质,使其对电子和能量转换设备以及作为电化学传感器的(电)催化剂具有吸引力。除了 AuNPs 的大小和形状之外,AuNP 传感器的电催化性能还取决于其合成中使用的稳定剂。在这里,AuNPs 与柠檬酸盐、藻酸盐和槲皮素合成,获得球形和带负电荷的纳米粒子。AuNPs用于修饰玻碳电极(AuNPs/GCE),通过扫描电子显微镜和电化学技术对其进行表征。根据稳定剂的不同,AuNPs/GCE 在电极表面显示出不同尺寸和分散程度的聚集体。AuNP 的聚集体影响薄膜的均匀性,电极的重现性及其在缓冲溶液中的响应。最后,为了评估 AuNPs/GCE 的电催化能力,我们研究了两种具有相反电荷的分析物的氧化:(1)日落黄(负)和(2)肼(正)。与 GCE 相比,AuNPs/GCE 对肼显示出良好的电催化性能,根据所使用的 AuNP,将电流增加至 50%,并将电位移动近 400 mV。对于带负电的分析物,电流降低了 50%,并且没有观察到电位变化。因此,AuNPs 的电催化性能高度依赖于分析物的性质。(1)日落黄(阴性)和(2)肼(阳性)。与 GCE 相比,AuNPs/GCE 对肼显示出良好的电催化性能,根据所使用的 AuNP,将电流增加至 50%,并将电位移动近 400 mV。对于带负电的分析物,电流降低了 50%,并且没有观察到电位变化。因此,AuNPs 的电催化性能高度依赖于分析物的性质。(1)日落黄(阴性)和(2)肼(阳性)。与 GCE 相比,AuNPs/GCE 对肼显示出良好的电催化性能,根据所使用的 AuNP,将电流增加至 50%,并将电位移动近 400 mV。对于带负电的分析物,电流降低了 50%,并且没有观察到电位变化。因此,AuNPs 的电催化性能高度依赖于分析物的性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号