Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clinical trial design for local therapies for brain metastases: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group.
The Lancet ( IF 98.4 ) Pub Date : 2018-01-01 , DOI: 10.1016/s1470-2045(17)30692-7 Brian M Alexander 1 , Paul D Brown 2 , Manmeet S Ahluwalia 3 , Hidefumi Aoyama 4 , Brigitta G Baumert 5 , Susan M Chang 6 , Laurie E Gaspar 7 , Steven N Kalkanis 8 , David R Macdonald 9 , Minesh P Mehta 10 , Riccardo Soffietti 11 , John H Suh 12 , Martin J van den Bent 13 , Michael A Vogelbaum 3 , Jeffrey S Wefel 14 , Eudocia Q Lee 15 , Patrick Y Wen 15 ,
The Lancet ( IF 98.4 ) Pub Date : 2018-01-01 , DOI: 10.1016/s1470-2045(17)30692-7 Brian M Alexander 1 , Paul D Brown 2 , Manmeet S Ahluwalia 3 , Hidefumi Aoyama 4 , Brigitta G Baumert 5 , Susan M Chang 6 , Laurie E Gaspar 7 , Steven N Kalkanis 8 , David R Macdonald 9 , Minesh P Mehta 10 , Riccardo Soffietti 11 , John H Suh 12 , Martin J van den Bent 13 , Michael A Vogelbaum 3 , Jeffrey S Wefel 14 , Eudocia Q Lee 15 , Patrick Y Wen 15 ,
Affiliation
|
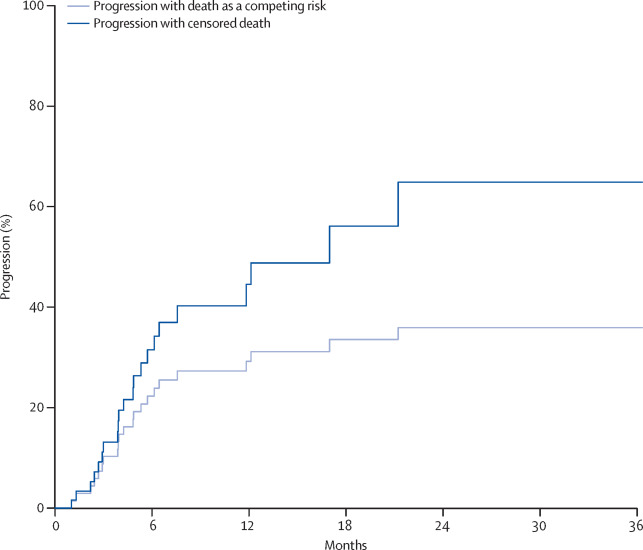
The goals of therapeutic and biomarker development form the foundation of clinical trial design, and change considerably from early-phase to late-phase trials. From these goals, decisions on specific clinical trial design elements, such as endpoint selection and statistical approaches, are formed. Whereas early-phase trials might focus on finding a therapeutic signal to make decisions on further development, late-phase trials focus on the confirmation of therapeutic impact by considering clinically meaningful endpoints. In this guideline from the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) working group, we highlight issues related to, and provide recommendations for, the design of clinical trials on local therapies for CNS metastases from solid tumours. We discuss endpoint selection criteria, the analysis appropriate for early-phase and late-phase trials, the association between tumour-specific and clinically meaningful endpoints, and possible issues related to the estimation of local control in the context of competing risks. In light of these discussions, we make specific recommendations on the clinical trial design of local therapies for brain metastases.
中文翻译:

脑转移瘤局部疗法的临床试验设计:《神经肿瘤脑转移瘤反应评估》指南。
治疗和生物标志物开发的目标构成了临床试验设计的基础,并且从早期阶段到后期阶段都有很大的变化。从这些目标出发,形成了针对特定临床试验设计要素的决策,例如终点选择和统计方法。早期试验可能专注于寻找治疗信号以决定进一步的发展,而晚期试验则专注于通过考虑具有临床意义的终点来确认治疗效果。在来自神经肿瘤脑转移瘤反应评估(RANO-BM)工作组的本指南中,我们重点介绍与实体瘤中枢神经系统转移局部治疗的临床试验设计相关的问题并提供建议。我们讨论端点选择标准,适用于早期和晚期试验的分析,肿瘤特异性终点和临床意义终点之间的关联以及与竞争风险相关的局部控制估计相关的可能问题。根据这些讨论,我们对脑转移的局部疗法的临床试验设计提出了具体建议。
更新日期:2017-12-31
中文翻译:

脑转移瘤局部疗法的临床试验设计:《神经肿瘤脑转移瘤反应评估》指南。
治疗和生物标志物开发的目标构成了临床试验设计的基础,并且从早期阶段到后期阶段都有很大的变化。从这些目标出发,形成了针对特定临床试验设计要素的决策,例如终点选择和统计方法。早期试验可能专注于寻找治疗信号以决定进一步的发展,而晚期试验则专注于通过考虑具有临床意义的终点来确认治疗效果。在来自神经肿瘤脑转移瘤反应评估(RANO-BM)工作组的本指南中,我们重点介绍与实体瘤中枢神经系统转移局部治疗的临床试验设计相关的问题并提供建议。我们讨论端点选择标准,适用于早期和晚期试验的分析,肿瘤特异性终点和临床意义终点之间的关联以及与竞争风险相关的局部控制估计相关的可能问题。根据这些讨论,我们对脑转移的局部疗法的临床试验设计提出了具体建议。











































 京公网安备 11010802027423号
京公网安备 11010802027423号