Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Valence‐Dependent Electrocatalytic Activity for Oxygen Evolution Reaction: A Case of Nickel Sulfides Hybridized with N and S Co‐Doped Carbon Nanoparticles
Small ( IF 13.0 ) Pub Date : 2017-12-29 , DOI: 10.1002/smll.201703273 Hongchao Yang 1, 2 , Changhong Wang 1, 2 , Yejun Zhang 1 , Qiangbin Wang 1, 2
Small ( IF 13.0 ) Pub Date : 2017-12-29 , DOI: 10.1002/smll.201703273 Hongchao Yang 1, 2 , Changhong Wang 1, 2 , Yejun Zhang 1 , Qiangbin Wang 1, 2
Affiliation
|
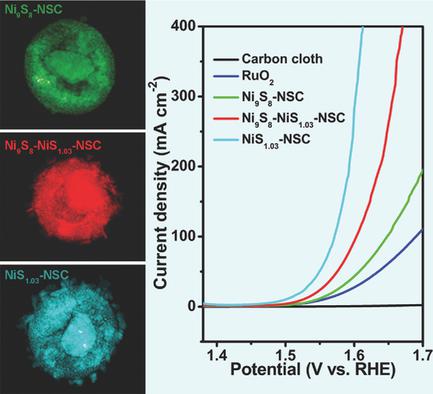
Exploration of the relationship between electrocatalytic activities and their chemical valence is very important in rational design of high‐efficient electrocatalysts. A series of porous nickel sulfides hybridized with N and S co‐doped carbon nanoparticles (NixSy‐NSCs) with different chemical valences of Ni, Ni9S8‐NSCs, Ni9S8‐NiS1.03‐NSCs, and NiS1.03‐NSCs are successfully fabricated, and their electrocatalytic performances as oxygen evolution reaction electrocatalysts are systematically investigated. The NixSy‐NSCs are obtained via a two‐step reaction including a low‐temperature synthesis of Ni‐Cys precursor followed by thermal decomposing of the precursor in Ar atmosphere. By controlling the sulfidation process during the formation of NixSy‐NSCs, Ni9S8‐NSCs, Ni9S8‐NiS1.03‐NSCs, and NiS1.03‐NSCs are obtained, respectively, giving rise to the increase of high‐valence Ni component, and resulting in gradually enhanced oxygen evolution reaction electrocatalytic activities. In particular, the NiS1.03‐NSCs show an exceptional low overpotential of ≈270 mV versus reversible hydrogen electrode at a current density of 10 mA cm−2 and a small Tafel slope of 68.9 mV dec−1 with mass loading of 0.25 mg cm−2 in 1 m KOH and their catalytic activities remained for at least 10 h, which surpass the state‐of‐the‐art IrO2, RuO2, and Ni‐based electrocatalysts.
中文翻译:

氧分解反应的化学价依赖性电催化活性:一例硫化镍与N和S共掺杂的碳纳米粒子杂化的情况
在合理设计高效电催化剂中,探索电催化活性与其化学价之间的关系非常重要。一系列多孔的硫化镍与N和S共掺杂的碳纳米粒子(Ni x S y -NSCs)杂化,这些纳米粒子具有不同的化学价态的Ni,Ni 9 S 8- NSCs,Ni 9 S 8 -NiS 1.03 - NSCs和NiS成功地制造了1.03 ‐NSC,并系统地研究了它们作为氧释放反应电催化剂的电催化性能。Ni x S y‐NSC是通过两步反应获得的,包括低温合成Ni-Cys前体,然后在Ar气氛中热分解前体。通过Ni的形成期间控制所述硫化过程X小号ÿ -NSCs,镍9小号8个-NSCs,镍9小号8 -NiS 1.03 -NSCs和NIS 1.03 -NSCs获得,分别,引起高的增加价镍成分,并导致逐渐增强的析氧反应电催化活性。特别是NiS 1.03-NSCs显示以10mA cm 2的电流密度的出色的低超电势≈270毫伏对可逆氢电极的-2和68.9毫伏癸小塔菲尔斜率-1 0.25毫克厘米质量负载-2 1米KOH和它们的催化活性至少持续了10个小时,超过了最新的IrO 2,RuO 2和Ni基电催化剂。
更新日期:2017-12-29
中文翻译:

氧分解反应的化学价依赖性电催化活性:一例硫化镍与N和S共掺杂的碳纳米粒子杂化的情况
在合理设计高效电催化剂中,探索电催化活性与其化学价之间的关系非常重要。一系列多孔的硫化镍与N和S共掺杂的碳纳米粒子(Ni x S y -NSCs)杂化,这些纳米粒子具有不同的化学价态的Ni,Ni 9 S 8- NSCs,Ni 9 S 8 -NiS 1.03 - NSCs和NiS成功地制造了1.03 ‐NSC,并系统地研究了它们作为氧释放反应电催化剂的电催化性能。Ni x S y‐NSC是通过两步反应获得的,包括低温合成Ni-Cys前体,然后在Ar气氛中热分解前体。通过Ni的形成期间控制所述硫化过程X小号ÿ -NSCs,镍9小号8个-NSCs,镍9小号8 -NiS 1.03 -NSCs和NIS 1.03 -NSCs获得,分别,引起高的增加价镍成分,并导致逐渐增强的析氧反应电催化活性。特别是NiS 1.03-NSCs显示以10mA cm 2的电流密度的出色的低超电势≈270毫伏对可逆氢电极的-2和68.9毫伏癸小塔菲尔斜率-1 0.25毫克厘米质量负载-2 1米KOH和它们的催化活性至少持续了10个小时,超过了最新的IrO 2,RuO 2和Ni基电催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号