当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium p-cymene iminophosphonamide complexes: activation under basic conditions and transfer hydrogenation catalysis
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2018-02-20 , DOI: 10.1002/ejic.201701344 Iana S. Sinopalnikova 1, 2 , Tatyana A. Peganova 2 , Natalia V. Belkova 2 , Eric Deydier 1 , Jean-Claude Daran 1 , Elena S. Shubina 2 , Alexander M. Kalsin 2 , Rinaldo Poli 1, 3
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2018-02-20 , DOI: 10.1002/ejic.201701344 Iana S. Sinopalnikova 1, 2 , Tatyana A. Peganova 2 , Natalia V. Belkova 2 , Eric Deydier 1 , Jean-Claude Daran 1 , Elena S. Shubina 2 , Alexander M. Kalsin 2 , Rinaldo Poli 1, 3
Affiliation
|
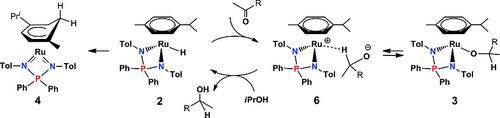
Complex [(η6-Cym)RuCl(NPN)] {Cym = p-cymene; NPN = (pTolN)2PPh2} (1) yields a thermally sensitive hydride derivative [(η6-Cym)RuH(NPN)] (2) by reaction with iPrOH in the presence of a strong base, via an observable isopropoxide intermediate [(η6-Cym)Ru(OiPr)(NPN)] (3), or with NaBHEt3 in toluene. Partial conversion also occurs in iPrOH in the absence of base. 2 is stabilized by dihydrogen bonding with isopropyl alcohol, but attempts to isolate it induce isomerization by hydride migration to a ring CH position to yield a 16-electron cyclohexadienyl derivative [{η5-p-C6H5(Me)(iPr)}Ru(NPN)], which has been crystallographically characterized as a disordered mixture of two regioisomers (4/4′). Complex 2 is able to release H2 upon treatment with medium strength proton donors (fluorinated alcohols), but also slowly with iPrOH. 2 is an active catalyst for the transfer hydrogenation of acetophenone to phenylethanol in isopropyl alcohol. The catalytic transformation is first order in acetophenone and first order in catalyst, with k = 117 ± 10 m–1 h–1 at 40 °C. The temperature dependence of the rate constant (25–80 °C) gave the activation parameters ΔH‡ = 9.6 ± 1.3 kcal mol–1 and ΔS‡ = –31 ± 4 cal mol–1 K–1. DFT calculations have validated the slow isomerization of 2 to 4/4′ (high energy TS), the preference of the cyclohexadienyl system for 4/4′ relative to the other isomers 4Me and 4iPr, where the hydride has migrated to the CMe or CiPr position, and suggest that the hydrogen transfer mechanism involves outer sphere hydride transfer to the ketone substrate with H-bonding assistance of isopropyl alcohol to yield a σ complex intermediate [(η6-Cym)Ru+(NPN){H-C(Me)(Ph)O–}].
中文翻译:

钌对伞花烃亚氨基磷酰胺配合物:碱性条件下的活化和转移氢化催化
络合物 [(η6-Cym)RuCl(NPN)] {Cym = p-cymene; NPN = (pTolN)2PPh2} (1) 通过可观察到的异丙醇中间体 [(η6 -Cym)Ru(OiPr)(NPN)] (3),或在甲苯中使用 NaBHEt3。在不存在碱的情况下,在 iPrOH 中也会发生部分转化。2 通过与异丙醇的二氢键合而稳定,但试图通过氢化物迁移到环 CH 位置诱导异构化以产生 16 电子环己二烯基衍生物 [{η5-p-C6H5(Me)(iPr)}Ru(NPN )],其在晶体学上被表征为两种区域异构体 (4/4') 的无序混合物。在用中等强度的质子供体(氟化醇)处理后,复合物 2 能够释放 H2,但也可以用 iPrOH 缓慢释放。2是苯乙酮在异丙醇中转移加氢生成苯乙醇的活性催化剂。催化转化是苯乙酮的一级和催化剂的一级,在 40 °C 下 k = 117 ± 10 m–1 h–1。速率常数 (25–80 °C) 的温度依赖性给出了活化参数 ΔH‡ = 9.6 ± 1.3 kcal mol–1 和 ΔS‡ = –31 ± 4 cal mol–1 K–1。DFT 计算验证了 2 到 4/4'(高能 TS)的缓慢异构化,相对于其他异构体 4Me 和 4iPr,环己二烯基系统对 4/4' 的偏好,其中氢化物已迁移到 CMe 或 CiPr位置,并表明氢转移机制涉及在异丙醇的氢键辅助下将外球氢化物转移到酮底物,以产生 σ 复合中间体 [(η6-Cym)Ru+(NPN){HC(Me)(Ph) O-}]。
更新日期:2018-02-20
中文翻译:

钌对伞花烃亚氨基磷酰胺配合物:碱性条件下的活化和转移氢化催化
络合物 [(η6-Cym)RuCl(NPN)] {Cym = p-cymene; NPN = (pTolN)2PPh2} (1) 通过可观察到的异丙醇中间体 [(η6 -Cym)Ru(OiPr)(NPN)] (3),或在甲苯中使用 NaBHEt3。在不存在碱的情况下,在 iPrOH 中也会发生部分转化。2 通过与异丙醇的二氢键合而稳定,但试图通过氢化物迁移到环 CH 位置诱导异构化以产生 16 电子环己二烯基衍生物 [{η5-p-C6H5(Me)(iPr)}Ru(NPN )],其在晶体学上被表征为两种区域异构体 (4/4') 的无序混合物。在用中等强度的质子供体(氟化醇)处理后,复合物 2 能够释放 H2,但也可以用 iPrOH 缓慢释放。2是苯乙酮在异丙醇中转移加氢生成苯乙醇的活性催化剂。催化转化是苯乙酮的一级和催化剂的一级,在 40 °C 下 k = 117 ± 10 m–1 h–1。速率常数 (25–80 °C) 的温度依赖性给出了活化参数 ΔH‡ = 9.6 ± 1.3 kcal mol–1 和 ΔS‡ = –31 ± 4 cal mol–1 K–1。DFT 计算验证了 2 到 4/4'(高能 TS)的缓慢异构化,相对于其他异构体 4Me 和 4iPr,环己二烯基系统对 4/4' 的偏好,其中氢化物已迁移到 CMe 或 CiPr位置,并表明氢转移机制涉及在异丙醇的氢键辅助下将外球氢化物转移到酮底物,以产生 σ 复合中间体 [(η6-Cym)Ru+(NPN){HC(Me)(Ph) O-}]。











































 京公网安备 11010802027423号
京公网安备 11010802027423号