Acta Biomaterialia ( IF 9.4 ) Pub Date : 2017-12-30 , DOI: 10.1016/j.actbio.2017.12.039 Ethan M Lotz 1 , Michael B Berger 1 , Zvi Schwartz 2 , Barbara D Boyan 3
|
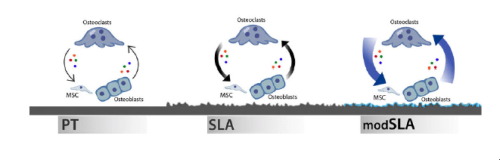
A critical stage during osseointegration of a titanium (Ti) implant is primary bone remodeling, which involves cross talk among osteoclast precursors, osteoclasts, mesenchymal stem cells (MSCs), and osteoblasts. This phase couples the processes of bone formation and resorption. During remodeling, osteoclasts produce factors capable of regulating MSC migration and osteogenesis. Furthermore, they degrade primary bone, creating a foundation with a specific chemistry, stiffness, and morphology for osteoblasts to synthesize and calcify their matrix. MSCs and osteoblasts receiving cues from the implant surface produce factors capable of regulating osteoclasts in order to promote net new bone formation. The purpose of this study was to determine the effects Ti implant surfaces have on bone remodeling. Human MSCs and normal human osteoblasts (NHOsts) were cultured separately on 15mm grade 2 smooth PT, hydrophobic-microrough SLA, hydrophilic-microrough Ti (mSLA) (Institut Straumann AG, Basel, Switzerland), or tissue culture polystyrene (TCPS). After 7d, conditioned media from surface cultures were used to treat human osteoclasts for 2d. Activity was measured by fluorescence of released collagen followed by mRNA quantification. This study demonstrates that MSC and NHOst cultures are able to suppress osteoclast activity in a surface dependent manner and osteoclast mRNA levels are selectively regulated by surface treatments. The substrate-dependent regulatory effect was mitigated when MSCs were silenced for integrin subunits and when conditioned media were denatured. These results indicate that MSCs and NHOsts regulate at least two aspects of remodeling: reduced fusion of new osteoclasts and reduced activity of existing osteoclasts.
Statement of Significance
In this study, we developed a novel in vitro model to study how microstructured and hydrophilic titanium implants impact bone remodeling for dental and orthopaedic applications. Our approach intersects biomaterials and systems physiology, revealing for the first time that implant surface properties are capable of regulating the communication among the cells involved in remodeling of primary bone during osseointegration. We believe that the basic research presented in our manuscript will provide important knowledge in our understanding of factors that impact implant success. Furthermore, it provides a solid foundation for the development of materials that enable rapid osseointegration and earlier loading times for implants in bone that has been compromised by trauma or disease.
中文翻译:

成骨细胞谱系细胞对破骨细胞的调节取决于钛植入物表面特性
钛 (Ti) 种植体骨整合过程中的一个关键阶段是初级骨重塑,其中涉及破骨细胞前体、破骨细胞、间充质干细胞 (MSC) 和成骨细胞之间的交互作用。这个阶段耦合了骨形成和骨吸收的过程。在重塑过程中,破骨细胞产生能够调节 MSC 迁移和成骨的因子。此外,它们降解初级骨,为成骨细胞合成和钙化其基质创造具有特定化学、硬度和形态的基础。间充质干细胞和成骨细胞接收来自植入物表面的信号,产生能够调节破骨细胞的因子,以促进净新骨形成。本研究的目的是确定钛种植体表面对骨重塑的影响。人 MSC 和正常人成骨细胞 (NHOsts) 分别在 15mm 2 级光滑 PT、疏水性微粗糙 SLA、亲水微粗糙 Ti (mSLA)(Institut Straumann AG,巴塞尔,瑞士)或组织培养聚苯乙烯 (TCPS) 上培养。 7天后,使用表面培养物的条件培养基处理人破骨细胞2天。通过释放的胶原蛋白的荧光以及随后的 mRNA 定量来测量活性。这项研究表明,MSC 和 NHOst 培养物能够以表面依赖性方式抑制破骨细胞活性,并且破骨细胞 mRNA 水平受到表面处理的选择性调节。当 MSC 的整合素亚基沉默以及条件培养基变性时,底物依赖性调节作用会减弱。这些结果表明 MSC 和 NHOst 至少调节重塑的两个方面:减少新破骨细胞的融合和降低现有破骨细胞的活性。
重要性声明
在这项研究中,我们开发了一种新型体外模型来研究微结构和亲水性钛植入物如何影响牙科和骨科应用的骨重塑。我们的方法将生物材料和系统生理学相结合,首次揭示种植体表面特性能够调节骨整合过程中参与原发骨重塑的细胞之间的通讯。我们相信,我们手稿中提出的基础研究将为我们理解影响种植成功的因素提供重要的知识。此外,它还为开发材料奠定了坚实的基础,使受创伤或疾病损害的骨中的植入物能够快速骨整合和更早的加载时间。











































 京公网安备 11010802027423号
京公网安备 11010802027423号