Acta Biomaterialia ( IF 9.4 ) Pub Date : 2017-12-30 , DOI: 10.1016/j.actbio.2017.12.037 Diana G Soares 1 , Zhanpeng Zhang 2 , Fatma Mohamed 3 , Thomas W Eyster 3 , Carlos A de Souza Costa 4 , Peter X Ma 5
|
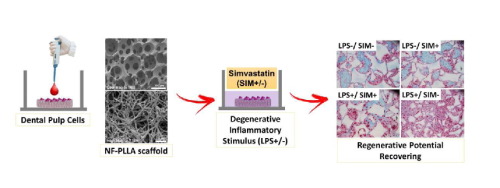
In this study, we investigated the anti-inflammatory, odontogenic and pro-angiogenic effects of integrating simvastatin and nanofibrous poly(l-lactic acid) (NF-PLLA) scaffolds on dental pulp cells (DPCs). Highly porous NF-PLLA scaffolds that mimic the nanofibrous architecture of extracellular matrix were first fabricated, then seeded with human DPCs and cultured with 0.1 μM simvastatin and/or 10 μg/mL pro-inflammatory stimulator lipopolysaccharide (LPS). The gene expression of pro-inflammatory mediators (TNF-α, IL-1β and MMP-9 mRNA) and odontoblastic markers (ALP activity, calcium content, DSPP, DMP-1 and BMP-2 mRNA) was quantified after long-term culture in vitro. In addition, we evaluated the scaffold’s pro-angiogenic potential after 24 hours of in vitro co-culture with endothelial cells. Finally, we assessed the combined effects of simvastatin and NF-PLLA scaffolds in vivo using a subcutaneous implantation mouse model. The in vitro studies demonstrated that, compared with the DPC/NF-PLLA scaffold constructs cultured only with pro-inflammatory stimulator LPS, adding simvastatin significantly repress the expression of pro-inflammatory mediators. Treating LPS+ DPC/NF-PLLA constructs with simvastatin also reverted the negative effects of LPS on expression of odontoblastic markers in vitro and in vivo. Western blot analysis demonstrated that these effects were related to a reduction in NFkBp65 phosphorylation and up-regulation of PPARγ expression, as well as to increased phosphorylation of pERK1/2 and pSmad1, mediated by simvastatin on LPS-stimulated DPCs. The DPC/NF-PLLA constructs treated with LPS/simvastatin also led to an increase in vessel-like structures, correlated with increased VEGF expression in both DPSCs and endothelial cells. Therefore, the combination of low dosage simvastatin and NF-PLLA scaffolds appears to be a promising strategy for dentin regeneration with inflamed dental pulp tissue, by minimizing the inflammatory reaction and increasing the regenerative potential of resident stem cells.
.
The regeneration potential of stem cells is dependent on their microenvironment. In this study, we investigated the effect of the microenvironment of dental pulp stem cells (DPSCs), including 3D structure of a macroporous and nanofibrous scaffold, the inflammatory stimulus lipopolysaccharide (LPS) and a biological molecule simvastatin, on their regenerative potential of mineralized dentin tissue. The results demonstrated that LPS upregulates inflammatory mediators and suppressed the odontogenic potential of DPSCs. Known as a lipid-lowing agent, simvastatin was excitingly found to repress the expression of pro-inflammatory mediators, up-regulate odontoblastic markers, and exert a pro-angiogenic effect on endothelial cells, resulting in enhanced vascularization and mineralized dentin tissue regeneration in a biomimetic 3D tissue engineering scaffold. This novel finding is significant for the fields of stem cells, inflammation and dental tissue regeneration.
中文翻译:

辛伐他汀和纳米纤维聚(l-乳酸)支架可在炎性环境中促进牙髓细胞的牙源性潜力。
在这项研究中,我们调查了辛伐他汀和纳米纤维聚(l-乳酸)(NF-PLLA)支架整合对牙髓细胞(DPC)的抗炎,牙源性和促血管生成作用。首先制造模仿细胞外基质的纳米纤维结构的高度多孔的NF-PLLA支架,然后将其接种人DPC,并用0.1μM辛伐他汀和/或10μg/ mL促炎性刺激剂脂多糖(LPS)培养。长期培养后,对促炎性介质(TNF-α,IL-1β和MMP-9 mRNA)和成牙本质标志物(ALP活性,钙含量,DSPP,DMP-1和BMP-2 mRNA)的基因表达进行定量。体外。此外,我们评估了体外24小时后支架的促血管生成潜力与内皮细胞共培养。最后,我们使用皮下植入小鼠模型评估了辛伐他汀和NF-PLLA支架在体内的联合作用。该体外研究表明,与DPC / NF-PLLA支架的构建体相比,具有促炎性刺激物LPS仅培养,加入辛伐他汀显著抑制促炎介质的表达。治疗LPS + DPC / NF-PLLA构建辛伐他汀也收归上成齿质细胞标志物的表达LPS的负面影响在体外和体内。蛋白质印迹分析表明,这些作用与辛伐他汀在LPS刺激的DPC上介导的NFkBp65磷酸化的减少和PPARγ表达的上调,以及pERK1 / 2和pSmad1的磷酸化增加有关。用LPS /辛伐他汀处理的DPC / NF-PLLA构建体还导致血管样结构的增加,与DPSCs和内皮细胞中VEGF表达的增加有关。因此,低剂量的辛伐他汀和NF-PLLA支架的组合通过最小化炎症反应并增加驻留干细胞的再生潜力,似乎是一种具有牙髓发炎的牙本质再生的有前途的策略。
。
干细胞的再生潜力取决于其微环境。在这项研究中,我们调查了牙髓干细胞(DPSCs)的微环境对矿化牙本质再生潜力的影响,包括大孔和纳米纤维支架的3D结构,炎性刺激脂多糖(LPS)和生物分子辛伐他汀。组织。结果表明,LPS上调了炎症介质,并抑制了DPSC的牙源性潜力。辛伐他汀被称为降脂剂,令人兴奋地被发现可抑制促炎性介质的表达,上调成牙本质细胞标志物并对内皮细胞发挥促血管生成作用,从而导致血管生成增强和矿化牙本质组织再生。仿生3D组织工程支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号