Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-buffered pH at carbon surfaces in aqueous supercapacitors
Carbon ( IF 10.5 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.carbon.2017.12.101 Adam Slesinski , Camelia Matei-Ghimbeu , Krzysztof Fic , François Béguin , Elzbieta Frackowiak
Carbon ( IF 10.5 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.carbon.2017.12.101 Adam Slesinski , Camelia Matei-Ghimbeu , Krzysztof Fic , François Béguin , Elzbieta Frackowiak
|
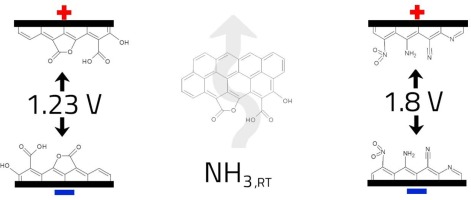
Abstract This paper presents a unique strategy aiming at extending the operational voltage range in supercapacitor working in the neutral aqueous electrolyte. In-depth analysis of the equilibria formed at the carbon/electrolyte interface gives the possibility to reach 1.8 V with excellent reversibility during cycling. To reach the goal, the donor - acceptor nature of carbon electrode surface has been taken into consideration. Controlled oxidation of electrode materials coupled with ammonia adsorption have been realized in order to adjust the optimal equilibrium conditions. Nitric acid oxidation of carbon leads to the formation of electrochemically active acidic sites promoting adsorption of ammonia. Modification with ammonia results in the introduction of self-controlled pH gradient within the capacitor system and formation of protective layer on electrodes, responsible for higher overpotentials of solvent decomposition. This, in turn, allows capacitor operation at higher voltages. Furthermore, this configuration eliminates the need for physical separation of ions (such as an ion-exchange membrane) to decelerate their mixing and improves the power density of the device.
中文翻译:

水性超级电容器中碳表面的自缓冲 pH 值
摘要 本文提出了一种独特的策略,旨在扩大在中性水性电解质中工作的超级电容器的工作电压范围。对碳/电解质界面处形成的平衡进行深入分析,可以在循环过程中达到 1.8 V 并具有出色的可逆性。为了达到这个目标,碳电极表面的供体-受体性质已被考虑在内。已实现电极材料的受控氧化与氨吸附相结合,以调整最佳平衡条件。碳的硝酸氧化导致形成促进氨吸附的电化学活性酸性位点。用氨改性导致在电容器系统内引入自控 pH 梯度并在电极上形成保护层,导致溶剂分解的过电位更高。这反过来又允许电容器在更高的电压下工作。此外,这种配置消除了对离子进行物理分离(例如离子交换膜)以减慢它们的混合并提高设备的功率密度的需要。
更新日期:2018-04-01
中文翻译:

水性超级电容器中碳表面的自缓冲 pH 值
摘要 本文提出了一种独特的策略,旨在扩大在中性水性电解质中工作的超级电容器的工作电压范围。对碳/电解质界面处形成的平衡进行深入分析,可以在循环过程中达到 1.8 V 并具有出色的可逆性。为了达到这个目标,碳电极表面的供体-受体性质已被考虑在内。已实现电极材料的受控氧化与氨吸附相结合,以调整最佳平衡条件。碳的硝酸氧化导致形成促进氨吸附的电化学活性酸性位点。用氨改性导致在电容器系统内引入自控 pH 梯度并在电极上形成保护层,导致溶剂分解的过电位更高。这反过来又允许电容器在更高的电压下工作。此外,这种配置消除了对离子进行物理分离(例如离子交换膜)以减慢它们的混合并提高设备的功率密度的需要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号