当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Raman Spectroscopy for Monitoring the Continuous Crystallization of Carbamazepine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.oprd.7b00322 David Acevedo 1 , Xiaochuan Yang 1 , Adil Mohammad 1 , Naresh Pavurala 1 , Wei-Lee Wu 1 , Thomas F. O’Connor 1 , Zoltan K. Nagy 2 , Celia N. Cruz 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.oprd.7b00322 David Acevedo 1 , Xiaochuan Yang 1 , Adil Mohammad 1 , Naresh Pavurala 1 , Wei-Lee Wu 1 , Thomas F. O’Connor 1 , Zoltan K. Nagy 2 , Celia N. Cruz 1
Affiliation
|
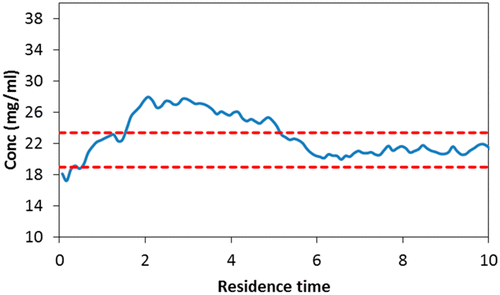
Crystallization has a significant impact on the properties of the active pharmaceutical ingredient (API) since it is the final step in the manufacturing of the drug substance and determines particle size distribution, purity, shape, and polymorphs. Many publications have focused on the implementation of Process Analytical Technology (PAT) tools for monitoring batch and continuous operation; however, a comprehensive method development and validation of Raman spectroscopy to monitor continuous crystallization has not been presented. This work demonstrates the development and validation of a method to monitor the solute concentration of Carbamazepine and quantifies the limit of detection for a metastable polymorphic form. The experiments were based on the cooling crystallization of Carbamazepine to produce the most stable form. The method was validated following the principles described in USP general chapter ⟨1225⟩ validation procedures for analytical methods. The results demonstrate the model can predict the solute concentration with a root-mean-square-error of prediction of 2.46 mg/mL. The repeatability and intermediate precision were evaluated, and the relative standard deviation is below 5%. The limit of detection for the metastable form was determined by monitoring the ratio of characteristic peaks when increasing the percentage of the metastable form in the total amount of crystals in the solution. A significant change in the peak ratio is observed when 22.9% of the crystals are of the metastable form. In addition, this PAT method was used to monitor a continuous run for 10 residence times, in which the system reached a controlled state of operation after six residence times.
中文翻译:

拉曼光谱法监测卡马西平的连续结晶
结晶对活性药物成分(API)的性质有重大影响,因为它是原料药生产中的最后一步,它决定了粒度分布,纯度,形状和多晶型物。许多出版物都集中在过程分析技术(PAT)工具的实施上,以监视批生产和连续操作。然而,尚未提出用于监测连续结晶的拉曼光谱的综合方法开发和验证。这项工作证明了监测卡马西平溶质浓度并量化亚稳多晶型物检出限的方法的开发和验证。实验基于卡马西平的冷却结晶以产生最稳定的形式。该方法已按照美国药典通则第1225章分析方法验证程序中所述的原则进行了验证。结果表明该模型可以预测溶质浓度,预测的均方根误差为2.46 mg / mL。评价了重复性和中间精度,相对标准偏差低于5%。当增加溶液中晶体总量中亚稳态形式的百分比时,可通过监测特征峰的比率来确定亚稳态形式的检出限。当22.9%的晶体为亚稳态形式时,观察到峰比率的显着变化。此外,此PAT方法还用于监控连续运行10个停留时间,
更新日期:2018-01-12
中文翻译:

拉曼光谱法监测卡马西平的连续结晶
结晶对活性药物成分(API)的性质有重大影响,因为它是原料药生产中的最后一步,它决定了粒度分布,纯度,形状和多晶型物。许多出版物都集中在过程分析技术(PAT)工具的实施上,以监视批生产和连续操作。然而,尚未提出用于监测连续结晶的拉曼光谱的综合方法开发和验证。这项工作证明了监测卡马西平溶质浓度并量化亚稳多晶型物检出限的方法的开发和验证。实验基于卡马西平的冷却结晶以产生最稳定的形式。该方法已按照美国药典通则第1225章分析方法验证程序中所述的原则进行了验证。结果表明该模型可以预测溶质浓度,预测的均方根误差为2.46 mg / mL。评价了重复性和中间精度,相对标准偏差低于5%。当增加溶液中晶体总量中亚稳态形式的百分比时,可通过监测特征峰的比率来确定亚稳态形式的检出限。当22.9%的晶体为亚稳态形式时,观察到峰比率的显着变化。此外,此PAT方法还用于监控连续运行10个停留时间,










































 京公网安备 11010802027423号
京公网安备 11010802027423号