当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
B(C6F5)3-Catalyzed C3-Selective C–H Borylation of Indoles: Synthesis, Intermediates, and Reaction Mechanism
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.joc.7b02886 Sutao Zhang 1 , Yuxi Han 1 , Jianghua He 1 , Yuetao Zhang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.joc.7b02886 Sutao Zhang 1 , Yuxi Han 1 , Jianghua He 1 , Yuetao Zhang 1
Affiliation
|
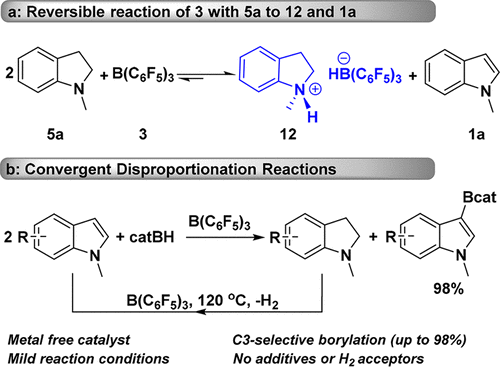
Without the addition of any additives and production of any small molecules, C3-borylated indoles and transfer hydrogenated indolines have been simultaneously achieved by a B(C6F5)3-catalyzed disproportionation reaction of a broad range of indoles with catecholborane. This catalyst system exhibits excellent catalytic performance for practical applications, such as easy scale-up under solvent-free conditions and long catalytic lifetime over ten sequential additions of starting materials. A combined mechanistic study, including isolation and characterization of key reaction intermediates, analysis of the disproportionation nature of the reaction, in situ NMR of the reaction, and analysis of detailed experimental data, has led to a possible reaction mechanism which illustrates pathways for the formation of both major products and byproducts. Understanding the reaction mechanism enables us to successfully suppress side reactions by choosing appropriate substrates and adjusting the amount of catecholborane needed. More importantly, with an elevated reaction temperature, we could achieve the convergent disproportionation reaction of indoles, in which indolines were continuously oxidized to indoles for the next disproportionation catalytic cycle. Near quantitative conversions and up to 98% yields of various C3-selective borylated indoles were achieved, without any additives or H2 acceptors.
中文翻译:

B(C 6 F 5)3-吲哚的C3选择性C–H硼化反应:合成,中间体和反应机理
在不添加任何添加剂和不产生任何小分子的情况下,通过B(C 6 F 5)3可同时实现C3硼化的吲哚和氢化氢化的二氢吲哚。儿茶酚硼烷催化多种吲哚催化歧化反应。该催化剂体系在实际应用中显示出优异的催化性能,例如在无溶剂条件下易于按比例放大,并且在十次连续添加起始原料的情况下具有长催化寿命。组合的机理研究,包括关键反应中间体的分离和表征,反应歧化性质的分析,反应的原位NMR以及详细的实验数据的分析,导致了可能的反应机理,该反应机理说明了形成途径主要产品和副产品的数量。了解反应机理使我们能够通过选择合适的底物并调节所需的儿茶酚硼烷量来成功抑制副反应。更重要的是,随着反应温度的升高,我们可以实现吲哚的收敛歧化反应,在该歧化反应中,二氢吲哚被连续氧化成吲哚,以进行下一个歧化催化循环。在没有任何添加剂或H的情况下,实现了接近定量的转化,各种C3选择性硼化的吲哚的收率高达98%。2个受体。
更新日期:2018-01-16
中文翻译:

B(C 6 F 5)3-吲哚的C3选择性C–H硼化反应:合成,中间体和反应机理
在不添加任何添加剂和不产生任何小分子的情况下,通过B(C 6 F 5)3可同时实现C3硼化的吲哚和氢化氢化的二氢吲哚。儿茶酚硼烷催化多种吲哚催化歧化反应。该催化剂体系在实际应用中显示出优异的催化性能,例如在无溶剂条件下易于按比例放大,并且在十次连续添加起始原料的情况下具有长催化寿命。组合的机理研究,包括关键反应中间体的分离和表征,反应歧化性质的分析,反应的原位NMR以及详细的实验数据的分析,导致了可能的反应机理,该反应机理说明了形成途径主要产品和副产品的数量。了解反应机理使我们能够通过选择合适的底物并调节所需的儿茶酚硼烷量来成功抑制副反应。更重要的是,随着反应温度的升高,我们可以实现吲哚的收敛歧化反应,在该歧化反应中,二氢吲哚被连续氧化成吲哚,以进行下一个歧化催化循环。在没有任何添加剂或H的情况下,实现了接近定量的转化,各种C3选择性硼化的吲哚的收率高达98%。2个受体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号