European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-12-29 , DOI: 10.1016/j.ejmech.2017.12.089 Atta Ullah , Fatima Iftikhar , Muhammad Arfan , Syeda Tayyaba Batool Kazmi , Muhammad Naveed Anjum , Ihsan-ul Haq , Muhammad Ayaz , Sadia Farooq , Umer Rashid
|
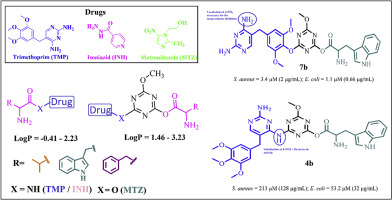
Present work describes the in vitro antibacterial evaluation of some new amino acid conjugated antimicrobial drugs. Structural modification was attempted on the three existing antimicrobial pharmaceuticals namely trimethoprim, metronidazole, isoniazid. Twenty one compounds from seven series of conjugates of these drugs were synthesized by coupling with some selected Boc-protected amino acids. The effect of structural features and lipophilicity on the antibacterial activity was investigated. The synthesized compounds were evaluated against five standard American type culture collection (ATCC) i.e. Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa and Salmonella typhi strains of bacteria. Our results identified a close relationship between the lipophilicity and the activity. Triazine skeleton proved beneficial for the increase in hydrophobicity and potency. Compounds with greater hydrophobicity have shown excellent activities against Gram-negative strains of bacteria than Gram-positive. 4-amino unsubstituted trimethoprim-triazine derivative 7b have shown superior activity with MIC = 3.4 μM (2 μg/mL) for S. aureus and 1.1 μM (0.66 μg/mL) for E. coli. The synthesized compounds were also evaluated for their urease inhibition study. Microbial urease from Bacillus pasteurii was chosen for this study. Triazine derivative 7a showed excellent inhibition with IC50 = 6.23 ± 0.09 μM. Docking studies on the crystal structure of B. pasteurii urease (PDB ID 4UBP) were carried out.
中文翻译:

氨基酸共轭抗菌药物:合成,亲脂活性关系,抗菌和脲酶抑制活性
目前的工作描述了一些新的氨基酸偶联抗菌药物的体外抗菌评价。尝试对三种现有的抗微生物药物进行了结构修饰,即甲氧苄啶,甲硝唑,异烟肼。通过与一些选定的受Boc保护的氨基酸偶联,合成了来自这些药物的七个共轭物的21个化合物。研究了结构特征和亲脂性对抗菌活性的影响。针对五个标准的美国典型培养物保藏中心(ATCC)评估了合成的化合物,即金黄色葡萄球菌,枯草芽孢杆菌,大肠杆菌,铜绿假单胞菌和伤寒沙门氏菌细菌菌株。我们的结果确定了亲脂性和活性之间的密切关系。三嗪骨架被证明对增加疏水性和效力是有益的。与革兰氏阳性菌相比,疏水性更高的化合物对革兰氏阴性菌表现出优异的活性。4-氨基未取代的甲氧苄氨嘧啶-三嗪衍生物7b对金黄色葡萄球菌和大肠杆菌的MIC = 3.4μM(2μg/ mL)和1.1μM(0.66μg/ mL)表现出优异的活性。还评估了合成的化合物的脲酶抑制研究。本研究选择了巴斯德巴斯德芽孢杆菌的微生物脲酶。三嗪衍生物7a对IC 50表现出优异的抑制作用 = 6.23±0.09μM。对巴斯德毕赤酵母脲酶(PDB ID 4UBP)的晶体结构进行了对接研究。










































 京公网安备 11010802027423号
京公网安备 11010802027423号