Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-30 , DOI: 10.1016/j.tet.2017.12.046 Pavel A. Fedyushin , Roman Yu. Peshkov , Elena V. Panteleeva , Evgeny V. Tretyakov , Irina V. Beregovaya , Yuri V. Gatilov , Vitalij D. Shteingarts
|
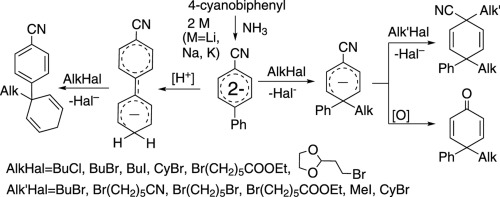
Birch's reductive alkylation of biphenyl-4-carbonitrile (1) provides alkylated 1,4-dihydroderivatives of various structural types: 4-alkyl-4-phenylcyclohexa-2,5-dienone, 1,4-dialkyl-4-phenylcyclohexa-2,5-dienecarbonitrile (with the same or different alkyl fragments), and 4-(1-alkylcyclohexa-2,5-dienyl)benzonitrile. Each of these products become dominant depending on the nature of long-living anionic form generated from 1, namely, the stable product of two-electrons reduction – dianion (12−); 1-alkyl-4-cyano-1-phenylcyclohexa-2,5-dien-4-yl anion (1-Alk1–), originated due to the alkylation of dianion 12− at the position 1 of biphenyl moiety; or 1-(4-cyanophenyl)cyclohexa-2,5-dien-1-yl anion (1-H4’–), being the product of dianion 12− protonation at position 4′ by protonating reagent (MeOH or NH4Cl). The orientation of alkyl fragment incorporation into biphenyl-4-carbonitrile scaffold is in agreement with calculated electronic structure of the anionic species under investigation. The dominating type of their reactivity towards alkyl halides proved to be nucleophilic (SN2 mechanism).
中文翻译:

有目的地的区域选择性控制联苯-4-腈的桦木还原烷基化
桦木对联苯-4-腈(1)的还原烷基化可提供各种结构类型的烷基化1,4-二氢衍生物:4-烷基-4-苯基环己-2,5-二烯酮,1,4-二烷基-4-苯基环己-2, 5-二烯腈(具有相同或不同的烷基片段)和4-(1-烷基环六-2,5-二烯基)苄腈。这些产物各自取决于1生成的长寿命阴离子形式的性质,即两电子还原的稳定产物–二价阴离子(1 2-); 1-烷基-4-氰基-1-苯基环己-2,5-二烯-4-基阴离子(1-Alk 1 –),是由于二价阴离子1 2−的烷基化而产生的在联苯部分的1位; 或1-(4-氰基苯基)环己-2,5-二烯-1-基阴离子(1-H 4' - ),作为二价阴离子的产物1 2-第4位的质子化'由质子化试剂(MeOH或NH 4 Cl)。烷基片段并入联苯-4-腈支架的取向与所研究的阴离子物质的计算电子结构一致。它们对烷基卤化物反应性的主导类型被证明是亲核(小号Ñ 2机构)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号