Molecular Therapy - Nucleic Acids ( IF 8.8 ) Pub Date : 2017-12-30 , DOI: 10.1016/j.omtn.2017.12.013 Weronika Kotkowiak 1 , Jolanta Lisowiec-Wachnicka 1 , Jakub Grynda 2 , Ryszard Kierzek 3 , Jesper Wengel 4 , Anna Pasternak 1
|
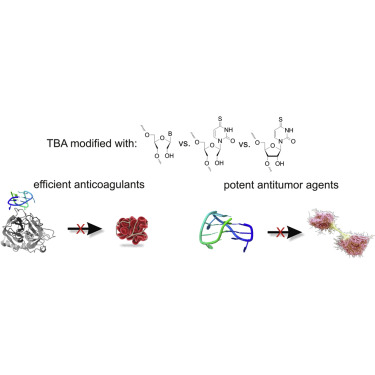
Thrombin is a serine protease that plays a crucial role in hemostasis, fibrinolysis, cell proliferation, and migration. Thrombin binding aptamer (TBA) is able to inhibit the activity of thrombin molecule via binding to its exosite I. This 15-nt DNA oligonucleotide forms an intramolecular, antiparallel G-quadruplex structure with a chair-like conformation. In this paper, we report on our investigations on the influence of certain modified nucleotide residues on thermodynamic stability, folding topology, and biological properties of TBA variants. In particular, the effect of single incorporation of a novel 4-thiouracil derivative of unlocked nucleic acid (UNA), as well as single incorporation of 4-thiouridine and all four canonical UNAs, was evaluated. The studies presented herein have shown that 4-thiouridine in RNA and UNA series, as well as all four canonical UNAs, can efficiently modulate G-quadruplex thermodynamic and biological stability, and that the effect is strongly position dependent. Interestingly, TBA variants containing the modified nucleotide residues are characterized by unchanged folding topology. Thrombin time assay revealed that incorporation of certain UNA residues may improve G-quadruplex anticoagulant properties. Noteworthy, some TBA variants, characterized by decreased ability to inhibit thrombin activity, possess significant antiproliferative properties reducing the viability of the HeLa cell line even by 95% at 10 μM concentration.
中文翻译:

含有新型UNA衍生物的凝血酶结合适体的热力学,抗凝血和抗增殖特性。
凝血酶是一种丝氨酸蛋白酶,在止血,纤维蛋白溶解,细胞增殖和迁移中起关键作用。凝血酶结合适体(TBA)能够通过结合其异位酶I抑制凝血酶分子的活性。该15-nt DNA寡核苷酸形成具有椅子样构象的分子内,反平行的G-四链体结构。在本文中,我们报告了我们对某些修饰核苷酸残基对TBA变体的热力学稳定性,折叠拓扑和生物学特性的影响的研究报告。特别地,评估了单次引入新的未锁定核酸的4-硫尿嘧啶衍生物(UNA),以及单次引入4-硫尿苷和所有四个规范性UNA的效果。本文介绍的研究表明,RNA和UNA系列中的4-硫尿苷 以及所有四个规范的UNA,都可以有效地调节G-四链体的热力学和生物稳定性,并且这种作用与位置密切相关。有趣的是,含有修饰的核苷酸残基的TBA变体的特征在于不变的折叠拓扑。凝血酶时间测定法显示某些UNA残基的掺入可以改善G四联体的抗凝特性。值得注意的是,某些TBA变体的特征在于抑制凝血酶活性的能力降低,它们具有显着的抗增殖特性,即使在浓度为10μM时,HeLa细胞系的活力也降低了95%。包含修饰的核苷酸残基的TBA变体的特征在于不变的折叠拓扑。凝血酶时间测定法显示某些UNA残基的掺入可以改善G四联体的抗凝特性。值得注意的是,某些TBA变体的特征在于抑制凝血酶活性的能力降低,它们具有显着的抗增殖特性,即使在浓度为10μM时,HeLa细胞系的活力也降低了95%。包含修饰的核苷酸残基的TBA变体的特征在于不变的折叠拓扑。凝血酶时间测定法显示某些UNA残基的掺入可以改善G四联体的抗凝特性。值得注意的是,某些TBA变体的特征在于抑制凝血酶活性的能力降低,它们具有显着的抗增殖特性,即使在浓度为10μM时,HeLa细胞系的活力也降低了95%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号