Cellular Signalling ( IF 4.4 ) Pub Date : 2017-12-24 , DOI: 10.1016/j.cellsig.2017.12.006 Fiona Haxho , Sabah Haq , Myron R. Szewczuk
|
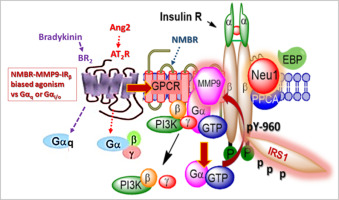
G protein-coupled receptors (GPCR) can participate in a number of signaling pathways, and this property led to the concept of biased GPCR agonism. Agonists, antagonists and allosteric modulators can bind to GPCRs in different ways, creating unique conformations that differentially modulate signaling through one or more G proteins. A unique neuromedin B (NMBR) GPCR-signaling platform controlling mammalian neuraminidase-1 (Neu1) and matrix metalloproteinase-9 (MMP9) crosstalk has been reported in the activation of the insulin receptor (IR) through the modification of the IR glycosylation. Here, we propose that there exists a biased GPCR agonism as small diffusible molecules in the activation of Neu1-mediated insulin receptor signaling. GPCR agonists bombesin, bradykinin, angiotensin I and angiotensin II significantly and dose-dependently induce Neu1 sialidase activity and IR activation in human IR-expressing rat hepatoma cell lines (HTC-IR), in the absence of insulin. Furthermore, the GPCR agonist-induced Neu1 sialidase activity could be specifically blocked by the NMBR inhibitor, BIM-23127. Protein expression analyses showed that these GPCR agonists significantly induced phosphorylation of IRβ and insulin receptor substrate-1 (IRS1). Among these, angiotensin II was the most potent GPCR agonist capable of promoting IRβ phosphorylation in HTC-IR cells. Interestingly, treatment with BIM-23127 and Neu1 inhibitor oseltamivir phosphate were able to block GPCR agonist-induced IR activation in HTC cells in vitro. Additionally, we found that angiotensin II receptor (type I) exists in a multimeric receptor complex with Neu1, IRβ and NMBR in naïve (unstimulated) and stimulated HTC-IR cells with insulin, bradykinin, angiotensin I and angiotensin II. This complex suggests a molecular link regulating the interaction and signaling mechanism between these molecules on the cell surface. These findings uncover a biased GPCR agonist-induced IR transactivation signaling axis, mediated by Neu1 sialidase and the modification of insulin receptor glycosylation.
中文翻译:

偏置的G蛋白偶联受体激动作用介导Neu1唾液酸酶和基质金属蛋白酶9串扰,以诱导胰岛素受体信号转导
G蛋白偶联受体(GPCR)可以参与许多信号传导途径,并且这种特性导致了GPCR激动剂有偏性的概念。激动剂,拮抗剂和变构调节剂可以不同方式与GPCR结合,形成独特的构象,该构象可通过一种或多种G蛋白差异调节信号传导。在通过IR糖基化修饰激活胰岛素受体(IR)中,已经报道了控制哺乳动物神经氨酸酶(Neu1)和基质金属蛋白酶9(MMP9)串扰的独特神经调节素B(NMBR)GPCR信号平台。在这里,我们建议在Neu1介导的胰岛素受体信号转导的激活中存在作为小的可扩散分子的偏向性GPCR激动作用。GPCR激动剂bombesin,缓激肽,在没有胰岛素的情况下,血管紧张素I和血管紧张素II在表达人IR的大鼠肝癌细胞系(HTC-IR)中显着且剂量依赖性地诱导Neu1唾液酸酶活性和IR活化。此外,NMBR抑制剂BIM-23127可以特异性阻断GPCR激动剂诱导的Neu1唾液酸酶活性。蛋白质表达分析表明,这些GPCR激动剂可显着诱导IRβ和胰岛素受体底物1(IRS1)的磷酸化。其中,血管紧张素II是最有效的GPCR激动剂,能够促进HTC-IR细胞中的IRβ磷酸化。有趣的是,用BIM-23127和Neu1抑制剂磷酸奥司他韦进行治疗能够阻断HTC细胞中GPCR激动剂诱导的IR活化 在没有胰岛素的情况下。此外,NMBR抑制剂BIM-23127可以特异性阻断GPCR激动剂诱导的Neu1唾液酸酶活性。蛋白质表达分析表明,这些GPCR激动剂可显着诱导IRβ和胰岛素受体底物1(IRS1)的磷酸化。其中,血管紧张素II是最有效的GPCR激动剂,能够促进HTC-IR细胞中的IRβ磷酸化。有趣的是,用BIM-23127和Neu1抑制剂磷酸奥司他韦进行治疗能够阻断HTC细胞中GPCR激动剂诱导的IR活化 在没有胰岛素的情况下。此外,NMBR抑制剂BIM-23127可以特异性阻断GPCR激动剂诱导的Neu1唾液酸酶活性。蛋白质表达分析表明,这些GPCR激动剂可显着诱导IRβ和胰岛素受体底物1(IRS1)的磷酸化。其中,血管紧张素II是最有效的GPCR激动剂,能够促进HTC-IR细胞中的IRβ磷酸化。有趣的是,用BIM-23127和Neu1抑制剂磷酸奥司他韦进行治疗能够阻断HTC细胞中GPCR激动剂诱导的IR活化 血管紧张素II是最有效的GPCR激动剂,能够在HTC-IR细胞中促进IRβ磷酸化。有趣的是,用BIM-23127和Neu1抑制剂磷酸奥司他韦进行治疗能够阻断HTC细胞中GPCR激动剂诱导的IR活化 血管紧张素II是最有效的GPCR激动剂,能够在HTC-IR细胞中促进IRβ磷酸化。有趣的是,用BIM-23127和Neu1抑制剂磷酸奥司他韦进行治疗能够阻断HTC细胞中GPCR激动剂诱导的IR活化体外。此外,我们发现血管紧张素II受体(I型)与Neu1,IRβ和NMBR的多聚体受体复合体存在于未受刺激的未经刺激的刺激HTC-IR细胞中(未经刺激),并带有胰岛素,缓激肽,血管紧张素I和血管紧张素II。该复合物提示了调节细胞表面上这些分子之间的相互作用和信号传导机制的分子连接。这些发现揭示了由Neu1唾液酸酶和胰岛素受体糖基化修饰介导的偏向GPCR激动剂诱导的IR反式激活信号轴。











































 京公网安备 11010802027423号
京公网安备 11010802027423号