当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Catalysis of the Electrochemical Oxygen Evolution Reaction by Iron(III) Ions Adsorbed on Amorphous Cobalt Oxide
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-12-29 00:00:00 , DOI: 10.1021/acscatal.7b03509 Luo Gong 1 , Xin Yu Esther Chng 1 , Yonghua Du 2 , Shibo Xi 2 , Boon Siang Yeo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-12-29 00:00:00 , DOI: 10.1021/acscatal.7b03509 Luo Gong 1 , Xin Yu Esther Chng 1 , Yonghua Du 2 , Shibo Xi 2 , Boon Siang Yeo 1
Affiliation
|
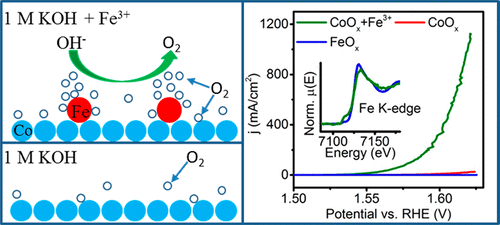
The oxygen evolution reaction (OER) is the bottleneck in the efficient production of hydrogen gas fuel via the electrochemical splitting of water. In this work, we present and elucidate the workings of an OER catalytic system which consists of cobalt oxide (CoOx) with adsorbed Fe3+ ions. The CoOx was electrodeposited onto glassy-carbon-disk electrodes, while Fe3+ was added to the 1 M KOH electrolyte. Linear sweep voltammetry and chronopotentiometry were used to assess the system’s OER activity. The addition of Fe3+ significantly lowered the average overpotential (η) required by the cobalt oxide catalyst to produce 10 mA/cm2 O2 current from 378 to 309 mV. The Tafel slope of the CoOx + Fe3+ catalyst also decreased from 59.5 (pure CoOx) to 27.6 mV/dec, and its stability lasted ∼20 h for 10 mA/cm2 O2 evolution. Cyclic voltammetry showed that oxidation of the deposited CoOx, from Co2+ to Co3+ occurred at a more positive potential when Fe3+ was added to the electrolyte. This could be attributed to interactions between the Co and Fe atoms. Comprehensive X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopy were conducted. The in situ XANES spectra of Co sites in the CoOx, CoOx + Fe3+, and control Fe48Co52Ox catalysts were similar during the OER, which indicates that the improved OER performance of the CoOx + Fe3+ catalyst could not be directly correlated to changes in the Co sites. The XANES spectra of Fe indicated that Fe3+ adsorbed on CoOx did not further oxidize under OER conditions. However, Fe’s coordination number was notably reduced from 6 in pure FeOx to 3.7 when it was adsorbed on CoOx. No change in the Fe–O bond lengths/strengths was found. The nature and mechanistic role of Fe adsorbed on CoOx are discussed. We propose that Fe sites with oxygen vacancies are responsible for the improved OER activity of CoOx + Fe3+ catalyst.
中文翻译:

吸附在非晶态氧化钴上的铁(III)离子对电化学氧分解反应的增强催化作用
氧气析出反应(OER)是通过水的电化学分解有效生产氢气燃料的瓶颈。在这项工作中,我们介绍并阐明了OER催化系统的工作原理,该系统由氧化钴(CoO x)和吸附的Fe 3+离子组成。将CoO x电沉积到玻璃碳圆盘电极上,同时将Fe 3+添加到1 M KOH电解质中。线性扫描伏安法和计时电位法用于评估系统的OER活性。Fe 3+的添加显着降低了钴氧化物催化剂产生10 mA / cm 2 O 2所需的平均超电势(η)电流从378至309 mV。CoO x + Fe 3+催化剂的Tafel斜率也从59.5(纯CoO x)降低到27.6 mV / dec,其稳定性持续约20 h,持续10 mA / cm 2 O 2释放。循环伏安法显示,当将Fe 3+添加到电解质中时,沉积的CoO x从Co 2+氧化为Co 3+发生氧化。这可以归因于Co和Fe原子之间的相互作用。进行了全面的X射线吸收近边缘结构(XANES)和扩展X射线吸收精细结构(EXAFS)光谱。CoO中Co位置的原位XANES光谱x,CoO x + Fe 3+和对照Fe 48 Co 52 O x催化剂在OER期间相似,这表明CoO x + Fe 3+催化剂改善的OER性能不能直接与Co的变化相关。网站。Fe的XANES光谱表明,在OER条件下,吸附在CoO x上的Fe 3+不会进一步氧化。然而,当Fe吸附在CoO x上时,其配位数从纯FeO x中的6显着降低至3.7。没有发现Fe-O键的长度/强度发生变化。Fe吸附在CoO上的性质和作用机理x被讨论。我们建议具有氧空位的Fe位点负责提高CoO x + Fe 3+催化剂的OER活性。
更新日期:2017-12-29
中文翻译:

吸附在非晶态氧化钴上的铁(III)离子对电化学氧分解反应的增强催化作用
氧气析出反应(OER)是通过水的电化学分解有效生产氢气燃料的瓶颈。在这项工作中,我们介绍并阐明了OER催化系统的工作原理,该系统由氧化钴(CoO x)和吸附的Fe 3+离子组成。将CoO x电沉积到玻璃碳圆盘电极上,同时将Fe 3+添加到1 M KOH电解质中。线性扫描伏安法和计时电位法用于评估系统的OER活性。Fe 3+的添加显着降低了钴氧化物催化剂产生10 mA / cm 2 O 2所需的平均超电势(η)电流从378至309 mV。CoO x + Fe 3+催化剂的Tafel斜率也从59.5(纯CoO x)降低到27.6 mV / dec,其稳定性持续约20 h,持续10 mA / cm 2 O 2释放。循环伏安法显示,当将Fe 3+添加到电解质中时,沉积的CoO x从Co 2+氧化为Co 3+发生氧化。这可以归因于Co和Fe原子之间的相互作用。进行了全面的X射线吸收近边缘结构(XANES)和扩展X射线吸收精细结构(EXAFS)光谱。CoO中Co位置的原位XANES光谱x,CoO x + Fe 3+和对照Fe 48 Co 52 O x催化剂在OER期间相似,这表明CoO x + Fe 3+催化剂改善的OER性能不能直接与Co的变化相关。网站。Fe的XANES光谱表明,在OER条件下,吸附在CoO x上的Fe 3+不会进一步氧化。然而,当Fe吸附在CoO x上时,其配位数从纯FeO x中的6显着降低至3.7。没有发现Fe-O键的长度/强度发生变化。Fe吸附在CoO上的性质和作用机理x被讨论。我们建议具有氧空位的Fe位点负责提高CoO x + Fe 3+催化剂的OER活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号