当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Highly Oxygenated Bisabolane Sesquiterpene Isolated from Ligularia lankongensis: Relative and Absolute Configurations of the Natural Product
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.joc.7b02688 Kenichi Kobayashi 1 , Risako Kunimura 1 , Hirokazu Takagi 2 , Misaki Hirai 2 , Hiroshi Kogen 1 , Hiroshi Hirota 3 , Chiaki Kuroda 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.joc.7b02688 Kenichi Kobayashi 1 , Risako Kunimura 1 , Hirokazu Takagi 2 , Misaki Hirai 2 , Hiroshi Kogen 1 , Hiroshi Hirota 3 , Chiaki Kuroda 2
Affiliation
|
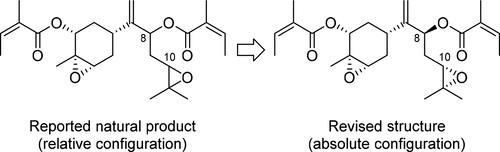
The relative and absolute configurations of an oxygenated bisabolane natural product, isolated from Ligularia lankongensis, were determined by synthesis. All four possible stereoisomers and their tiglate analogues were synthesized from R-(−)-carvone, and their 1H and 13C NMR spectra were compared to establish the 6R,8S,10S configuration. The stereoselective synthesis of the natural product was also achieved, featuring Brown allylation, vanadium-catalyzed epoxidation, and the Mitsunobu reaction.
中文翻译:

高度氧化Bisabolane倍半萜从隔离的全合成橐lankongensis天然产物的相对和绝对构型:
含氧bisabolane天然产品的相对和绝对构型中,从分离橐lankongensis,被合成来确定。从R -(-)-香芹酮合成了所有四种可能的立体异构体及其tiglate类似物,并比较了它们的1 H和13 C NMR光谱以建立6 R,8 S,10 S构型。还实现了天然产物的立体选择性合成,其特征在于布朗烯丙基化,钒催化的环氧化和Mitsunobu反应。
更新日期:2018-01-08
中文翻译:

高度氧化Bisabolane倍半萜从隔离的全合成橐lankongensis天然产物的相对和绝对构型:
含氧bisabolane天然产品的相对和绝对构型中,从分离橐lankongensis,被合成来确定。从R -(-)-香芹酮合成了所有四种可能的立体异构体及其tiglate类似物,并比较了它们的1 H和13 C NMR光谱以建立6 R,8 S,10 S构型。还实现了天然产物的立体选择性合成,其特征在于布朗烯丙基化,钒催化的环氧化和Mitsunobu反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号