Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of β-Amyloid Aggregation through a Designed β-Hairpin Peptide
Langmuir ( IF 3.7 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.langmuir.7b03617 Anjali Jha 1 , Mothukuri Ganesh Kumar 2 , Hosahudya N. Gopi 2 , Kishore M. Paknikar 1
Langmuir ( IF 3.7 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.langmuir.7b03617 Anjali Jha 1 , Mothukuri Ganesh Kumar 2 , Hosahudya N. Gopi 2 , Kishore M. Paknikar 1
Affiliation
|
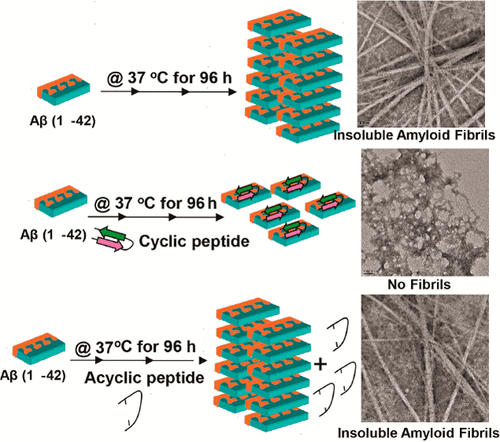
Designing peptide-based drugs to target the β-sheet-rich toxic intermediates during the aggregation of amyloid-β 1-42 (Aβ1-42) has been a major challenge. In general, β-sheet breaker peptides (BSBPs) are designed to complement the enthalpic interactions with the aggregating protein, and entropic effects are usually ignored. Here, we have developed a conformationally constrained cyclic BSBP by the use of an unnatural amino acid and a disulfide bond. We show that our peptide strongly inhibits the aggregation of Aβ1-42 in a concentration-dependent manner. It stabilizes the random coil conformation of Aβ1-42 monomers and inhibits the secondary structural transition to a β-sheet-rich conformation which allows Aβ1-42 to oligomerize in an ordered assembly during its aggregation. Our cyclic peptide also rescues the toxicity of soluble aggregates of Aβ1-42 toward neuronal cells. However, it significantly loses its potency in the conformationally relaxed acyclic form. It appears that limiting the loss of conformational entropy of the BSBP ligand can play a very important role in the attainment of conformations for precise and tight binding, making them a potent inhibitor for Aβ1-42 amyloidosis.
中文翻译:

通过设计的β-发夹肽抑制β-淀粉样蛋白聚集
设计基于肽的药物以在淀粉样蛋白β1-42(Aβ1-42)聚集期间靶向富含β-折叠的有毒中间体是一个重大挑战。通常,β-折叠肽(BSBP)被设计为与聚集蛋白互补焓相互作用,而熵效应通常被忽略。在这里,我们已经开发出通过使用非天然氨基酸和二硫键构象约束的环状BSBP。我们表明,我们的肽以浓度依赖性方式强烈抑制Aβ1-42的聚集。它可以稳定Aβ1-42单体的无规卷曲构象,并抑制二级结构转变为富含β-折叠的构象,从而允许Aβ1-42在聚集过程中以有序装配低聚。我们的环状肽还可以挽救Aβ1-42的可溶性聚集体对神经元细胞的毒性。但是,它以构象松弛的非环形式显着丧失了其效力。似乎,限制BSBP配体的构象熵的丧失可以在获得精确和紧密结合的构象中起非常重要的作用,使其成为Aβ1-42淀粉样变性的有效抑制剂。
更新日期:2018-01-16
中文翻译:

通过设计的β-发夹肽抑制β-淀粉样蛋白聚集
设计基于肽的药物以在淀粉样蛋白β1-42(Aβ1-42)聚集期间靶向富含β-折叠的有毒中间体是一个重大挑战。通常,β-折叠肽(BSBP)被设计为与聚集蛋白互补焓相互作用,而熵效应通常被忽略。在这里,我们已经开发出通过使用非天然氨基酸和二硫键构象约束的环状BSBP。我们表明,我们的肽以浓度依赖性方式强烈抑制Aβ1-42的聚集。它可以稳定Aβ1-42单体的无规卷曲构象,并抑制二级结构转变为富含β-折叠的构象,从而允许Aβ1-42在聚集过程中以有序装配低聚。我们的环状肽还可以挽救Aβ1-42的可溶性聚集体对神经元细胞的毒性。但是,它以构象松弛的非环形式显着丧失了其效力。似乎,限制BSBP配体的构象熵的丧失可以在获得精确和紧密结合的构象中起非常重要的作用,使其成为Aβ1-42淀粉样变性的有效抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号